2-dimensional materials have been successfully assembled into devices with the smallest possible man made holes for water desalination.
Researchers at the National Graphene Institute (NGI) at The University of Manchester have succeeded in fabricating tiny slits in a new membrane that are just several angstroms (0.1nm) in size. This has allowed the study of how various ions pass through these tiny holes.
The slits are made from graphene, hexagonal boron nitride (hBN) and molybdenum disulphide (MoS2) and, surprisingly, allow ions with diameters larger than the size of the slit to permeate through. The size-exclusion studies allow for a better understanding of how similar scale biological filters such as aquaporins work and so will help in the development of high-flux filters for water desalination and related technologies.
For scientists interested in the behaviour of fluids and their filtration, it has been an ultimate but seemingly distant goal to controllably fabricate capillaries with dimensions approaching the size of small ions and individual water molecules.
Researchers have been trying to mimic naturally-occurring ion transport systems, but this has proved to be no easy task. Channels fabricated with standard techniques and conventional materials have unfortunately been limited in size by the intrinsic roughness of a material’s surface, which is usually at least ten times bigger than the hydrated diameter of small ions.
Earlier this year graphene-oxide based membranes developed at the NGI attracted considerable attention as promising candidates for new filtration technologies. This research utilising the new toolkit of 2D materials demonstrates the real-world potential of providing clean drinking water from salt water.
To better understand the fundamental mechanisms behind ion transport, a team led by Sir Andre Geim of The University of Manchester made atomically flat slits measuring just several angstroms in size. These channels are chemically inert with smooth walls on the angstrom scale.
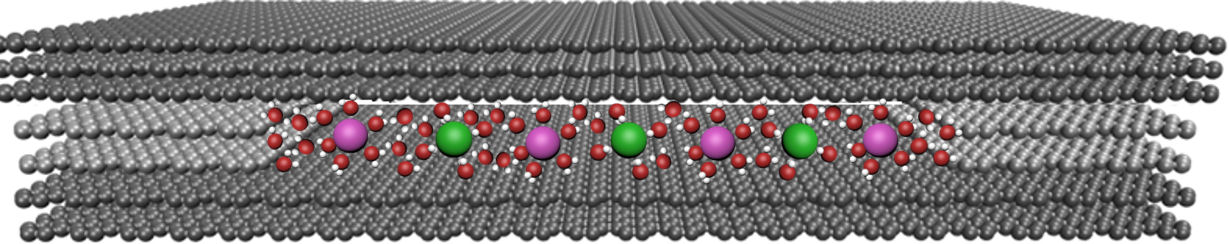
The researchers made their slit devices from two 100-nm thick crystal slabs of graphite measuring several microns across that they obtained by shaving off bulk graphite crystals. They then placed rectangular-shaped pieces of 2D atomic crystals of bilayer graphene and monolayer MoS2 at each edge of one of the graphite crystal slabs before placing another slab on top of the first. This produces a gap between the slabs that has a height equal to the spacers’ thickness.
“It’s like taking a book, placing two matchsticks on each of its edges and then putting another book on top.” explains Geim. “This creates a gap between the books’ surfaces with the height of the gap being equal to the matches’ thickness. In our case, the books are the atomically flat graphite crystals and the matchsticks are the graphene, or MoS2 monolayers.”
The assembly is held together by van der Waals forces and the slits are roughly the same size as the diameter of aquaporins, which are vital for living organisms. The slits are the smallest size possible since slits with thinner spacers are unstable and collapse because of attraction between opposite walls.
Ions flow through the slits if a voltage is applied across them when they are immersed in an ionic solution, and this ion flow constitutes an electric current. The team measured the ionic conductivity as they passed through chloride solutions via the slits and found that ions could move through them as expected under an applied electric field.
“When we looked more carefully, we found that bigger ions moved through more slowly than smaller ones like potassium chloride” explains Dr Gopi Kalon, a postdoctoral researcher who led the experimental effort.
Dr Ali Esfandiar, who is the first author of the paper, adds “The classical viewpoint is that ions with a diameter larger than the slit size cannot permeate, but our results show that this explanation is too simplistic. Ions in fact behave like soft tennis balls rather than hard billiard ones, and large ions can still pass – either by distorting their water shells or maybe shedding them altogether.
The new research as published in Science, shows that these newly observed mechanisms plays a key role for desalination using the size exclusion and is a key step to creating high-flux water desalination membranes.




