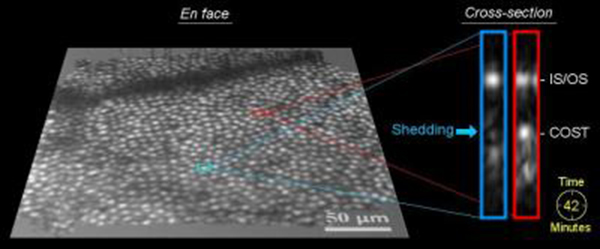
En face view shows the patch of cone photo-receptor cells that were studied in one of the subjects, approximately 1,000 cones. Each cone cell is distinguished by a bright punctuate reflection and spaced a few microns from neighboring cells. (Credit: Omer P. Kocaoglu, Indiana University)
An elusive biological cycle in the eye – the daily disposal and regeneration of the end tips of photoreceptor cells – has been captured in images for the first time in a living human eye. Photoreceptors are light-sensitive cells responsible for initiating vision. This glimpse into the inner workings of the eye will help scientists better understand, prevent and manage major eye diseases that affect photoreceptors like age-related macular degeneration and retinitis pigmentosa.
The physiological processes of disposal and regeneration—called “disc shedding” and “disc renewal”—are fundamental to maintaining the health of photoreceptors. The novel imaging method devised by these scientists detects disposal at the level of individual photoreceptor cells and clears a path for deeper explorations of vision and eye health.
The report on the collaborative work between Indiana University’s School of Optometry, Indiana and the University of California, Davis, Department of Ophthalmology and Vision Science, Calif., appears in the current issue of the journal of the Biomedical Optics Express.
Photoreceptors are conic or cylindrical structures that capture and convert light into an electrical response. The light, itself, is toxic as it leads to photo-oxidative compounds that would kill the cells if left to accumulate. To remain healthy, the cells must discard the membranes that contain the toxic compounds and then renew those that were lost. The difficulty lies in the fact that the cells have to maintain a constant length as they undergo this dynamic process each day. They cannot add too many renewing bits in the assembly process that the cell becomes too big, or too few that it becomes too small to work correctly.
“Shedding must be offset by renewal,” said Omer P. Kocaoglu, a biomedical engineer at Indiana University and the first author on the paper. “Dysfunction at any stage or loss in synchronization—such as loss of diurnal rhythm—can lead to photoreceptor and retinal pigment epithelium (RPE) dystrophy, and ultimately blindness.” The RPE is a thin but critically important layer of cells that nourishes and detoxifies photoreceptor cells.
“We and other imaging groups have been trying to detect this important physiological event for many years and have always come up short,” said Donald T. Miller, the lead researcher on the project. The end tips that the team is looking for are tiny – only a few microns in size – and are believed to break off the larger photoreceptor cell only once a day, after which they are quickly gobbled up by neighboring cells. This makes them difficult to capture.
“In some ways it is like looking for a needle in a haystack,” Miller said.
A key feature of their work is the discovery of the optical signature of this transient event as measured with their new imaging method. Their method combines two imaging technologies: adaptive optics, a technology first used in ground-based telescopes to correct aberrations induced by atmospheric turbulence, and optical coherence tomography (OCT), which makes cross-sectional images. Combined, the two methods provide exquisite optical resolution, permitting individual cells in the retina to be captured in all three dimensions – length, width and depth.
But the researchers wanted to take it a step further and actually track and monitor these cells over time – four dimensions. This required sophisticated post-processing algorithms, which the team developed. The team verified their observations with quantitative analyses of the spatial and temporal properties of the cone shedding dynamics in three healthy human subjects whose vision they were testing.
Previous research into this process, which has been taking place for decades, often used the eyes of dead animals for their model. Until the current study, no one knew exactly how this cycle was executed in humans.
“These first experiments establish a clear path for further investigation of photoreceptor shedding, which are now underway in our laboratory. Much awaits, and we have a great team to do it.” Miller added.




