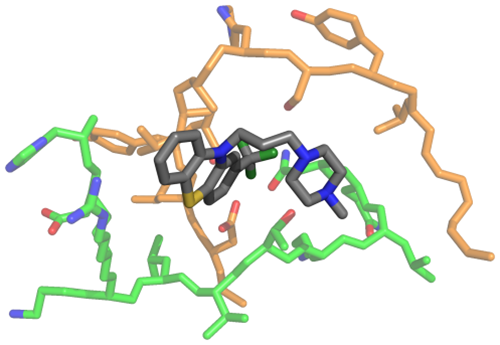
Molecular model of the drug (in gray) in interaction with some structures of the disordered proteins (in green and orange).
Pancreatic cancer is one of the most lethal tumors and, up until now, drugs available to fight it are only generic chemotherapy treatments. The protein Nupr1 belongs to the special class of “intrinsically disordered proteins” and its involvement in this pathology has been demonstrated since the ’90s by a French team of the National Institute of Health in Marseille.
The aim of discovering a molecule capable to inhibit this protein has been now reached through a study performed by the Institute of Nanotechnology of the National Research Council of Italy (Cnr-Nanotec), unit of Rende (Cosenza), in collaboration with several research units in Spain, including the universities of Elche and Zaragoza, and the center for hepato-digestive diseases of Madrid, in cooperation with the Cancer Center of Marseille.
“The research has been performed starting from the screening of more than 1000 drugs already approved for various therapeutic indications,” explains Bruno Rizzuti of Cnr-Nanotec in Rende. “The combined use of experimental techniques and computer simulations has allowed us to identify some of those drugs capable to interact with the protein Nupr1. ‘In vitro’ experiments have afterwards shown that the selected compounds were able to lower the vitality of tumor cells, reduce the ability of migration, and completely suppress the possibility of colony formation. The most effective compound has been tested ‘in vivo’ on human pancreatic cancer cells transplanted on mice, and proved to be able to completely arrest the development of the disease. The molecule at issue — known as trifluoperazine, and used until now only for its anti-psychotic action — has demonstrated an antitumor efficacy even higher than the most powerful chemotherapy treatments available so far. Furthermore, this study shows that this new molecule constitutes not only an alternative to such previously known drugs, but it can be combined with them to increase the overall therapeutic effect.”
“According to one of the dogmas of classical biology,” he adds, “the conformation of a protein should be unique and well defined to allow each of these ‘molecular machines’ to carry out a specific function. Disordered proteins overturn the validity of this principle and, due to their flexible structure, are able to perform multiple functions of cell communication and regulation. However, the absence of well-defined structural elements appeared to be an insurmountable obstacle to proceed to a rational design of selective drugs to hinder their action.”




