Researchers at Aalto University and Cambridge University have made a significant breakthrough in computational science by combining atomic-level modelling and machine learning. For the first time, the method has been used to realistically model how an amorphous material is formed at the atomic level: that is, a material that does not have a regular crystalline structure. The approach is expected to have impact on the research of many other materials.
“The secret of our success is machine learning, through which we can model the behavior of thousands of atoms over long periods of time. In this way, we have obtained a more accurate model,” says Postdoctoral Researcher Miguel Caro.
The team’s simulations reveal that diamond-like carbon film is formed at the atomic level in a different way than was thought. The prevailing understanding over the last 30 years of the formation mechanism for amorphous carbon film has been based on assumptions and indirect experimental results. Neither a good nor even an adequate atomic-level model has been available up to now. The new method has now overturned the earlier qualitative models and provided a precise atomic-level picture of the formation mechanism.
“Earlier, amorphous carbon films were thought to form when atoms are packed together in a small area. We have demonstrated that mechanical shock waves can cause the formation of diamond-like atoms further away from the point at which the impacting atoms hit the target,” reports Caro, who performed the simulations on CSC (IT Center for science) supercomputers, modelling the deposition of tens of thousands of atoms.
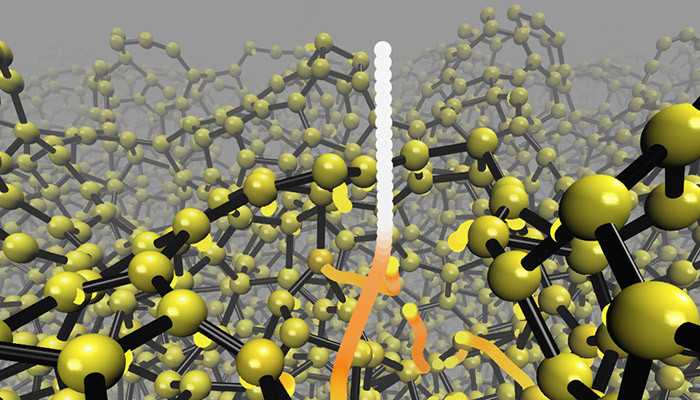
Trajectories followed by incident and knockon atoms during energetic deposition of a tetrahedral amorphous carbon thin film.
There are countless different uses for amorphous carbon. It is used as a coating in many mechanical applications, such as car motors, for example. In addition, the material can also be used for medical purposes and in various energy-related, biological, and environmental applications.
“For us, the most important application is biosensors. We have used very thin amorphous carbon coatings for identifying different biomolecules. In these applications, it is especially important to know the films’ electrical, chemical, and electrochemical properties and to be able to customize the material for a particular application,” explains Professor Tomi Laurila.
Dr. Volker Deringer, a Leverhulme Early Career Fellow, is particularly excited about using these methods for amorphous materials.
“‘Teaming up has been a great success,” say Deringer and Caro, who are continuing the collaboration between their institutions through ongoing visits. The team expect that their approach will help many others in experimental materials research, because it can give information about materials with a level of precision close to that of quantum mechanical methods, but simultaneously can make use of thousands of atoms and long simulation times. Both of these are extremely important for a realistic picture of the processes in experiments.
“I’m especially excited about the kinds of opportunities this method offers for further research. This atomic-level model produces verifiably correct results that correspond exceptionally well to the experimental results, revealing also for the first time the atomic-level phenomena behind the results. Using the model, we can, for example, predict what kind of carbon surface would be best for measuring neurotransmitters dopamine and serotonin,” says Laurila.
The research has been published in Physical Review Letters.
Source: Aalto University




