Every autumn, as the days grow shorter, an ancient foe―seasonal influenza―reemerges to cause morbidity and mortality. The most common influenza symptoms include fever, runny nose, sore throat, muscle pain, headache, coughing, and fatigue. While many people are able to recover from influenza, certain subpopulations (young children, pregnant women, and the elderly) face elevated risk of serious complications. Underscoring the burden of this disease, the United States Centers for Disease Control and Prevention estimates that influenza has resulted in between 9.2 million and 35.6 million illnesses, between 140,000 and 710,000 hospitalizations, and between 12,000 and 56,000 deaths annually since 2010.
Several factors complicate physicians’ ability to prevent and treat influenza outbreaks. To date, only two classes of drugs have been approved to treat influenza—M2 blockers (e.g., amantadine and rimantadine) and neuramindase inhibitors (e.g., oseltamivir and zanamivir). While influenza vaccines are a useful preventive measure, they are less effective in the elderly because their immune system tends to become less efficient with age. Furthermore, the rise of drug-resistant influenza strains and high rates of vaccine mismatches drive the search for new safe and effective antiviral drugs.
Modeling and simulation has been embraced by regulators, industry, and academia for its ability to inform antiviral drug development decision making. The modeling approaches employed include viral kinetic modeling, pharmacokinetic/pharmacodynamic (PK/PD) modeling, and pharmacoepidemiological/pharmacoeconomic modeling. Each approach offers distinct insights into influenza virology, pharmacology, and managing outbreaks.
Viral kinetic modeling: Characterizing the interplay between virus and host
The influenza virus infects epithelial cells in the respiratory tract. Infected cells produce new viruses, which infect more epithelial cells. Infected epithelial cells die, and the immune system clears the virus. In immunocompetent adults, the influenza virus generally results in a short infection. After infection, the virus replicates exponentially and the peak viral load occurs two to three days post-infection followed by an exponential decline.
Viral kinetic models use mathematical equations to describe the changes in viral load with time in an infected patient. They can provide important information about the cell infection rate, viral production rate, and viral clearance rate. For example, Researchers at the Los Alamos National Laboratory used a “target cell-limited model” that had been developed to understand other human viral pathogens to elucidate the interplay between the influenza virus and the immune system. To reproduce the viral titer peaks observed in patients, their model described the dynamics of uninfected target cells, productively infected cells, infectious viral titer, and the inhibition of viral replication by the innate host immune response.
Viral kinetic models have also helped identify new drug targets that efficiently interfere with viral replication. Researchers at the Max Planck Institute for Dynamics of complex Technical Systems used a multiscale modeling approach to describe influenza viral dynamics at both the intra- and extracellular levels. Their model suggested which steps in the viral life cycle might be most susceptible to pharmacological inhibition.
The use of viral kinetic models has played a critical role in expanding our knowledge of influenza virology and pathophysiology.
Combining PK/PD modeling with viral kinetic modeling
Viral kinetic models can also be combined with PK/PD models to explore how exposure to antiviral drugs reduces viral load and attenuates disease symptoms. In a 2015 study published in Antimicrobial Agents and Chemotherapy, researchers used PK/PD modeling to examine the impact of timing of antiviral administration on decreasing the duration of viral shedding. They found that early treatment with oseltamivir is likely to have the greatest impact on shortening the time in which patients shed virus and can infect others.
PK/PD models have also been used to describe the mechanisms behind the emergence and spread of drug resistance. For example, a research team based out of Los Alamos National Laboratory developed a model that included oseltamivir PK, symptom dynamics, and patient physiological variability. The findings from this model suggested that the timing of initiating prophylactic oseltamivir treatment was a primary factor in the emergence of drug resistance.
Taken together, these mechanistic models have helped guide the optimal use of antiviral drugs in various clinical influenza scenarios.
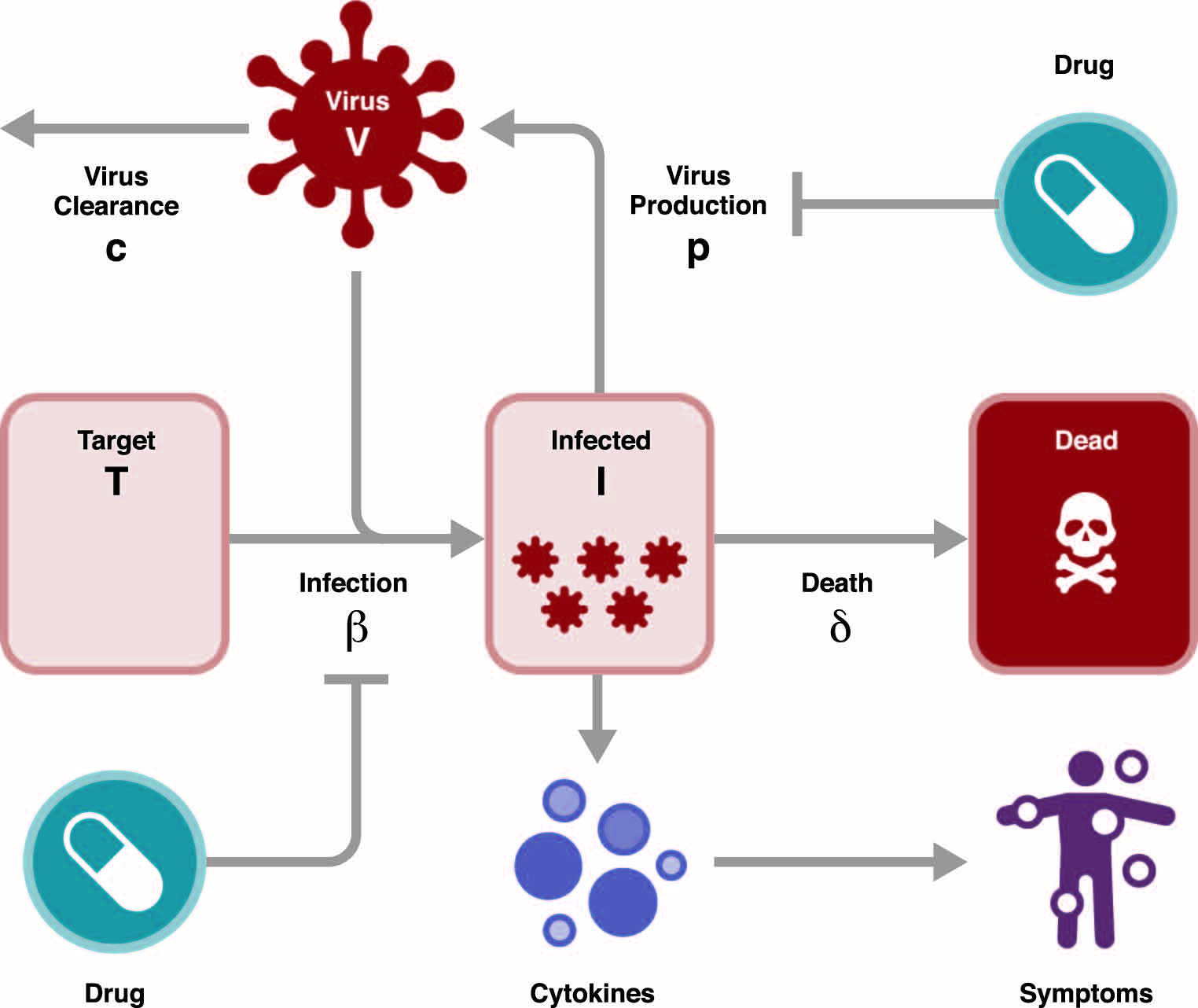
From the paper, Applications of Influenza Viral Kinetic Modeling in Drug Development, published in Current Pharmacology Reports. Credit: Mark Lovern, Ph.D.
Pharmacology to payer: One quantitative drug development framework to rule them all
Historically, infectious disease models were developed in discrete silos and the viral biology, anti-viral pharmacology, epidemiology, and health economic impacts were not linked. In recent years, an integrated, interdisciplinary approach has emerged that links antiviral PK/PD, epidemiology, and health economics.
Epidemiologic models can quantify changes in susceptible, exposed, infected, and recovered patients during an outbreak and assess the ease with which the influenza virus can be transmitted among them. While extremely powerful, epidemiology models typically make simplistic assumptions regarding variability in viral dynamics and antiviral drug activity, including the impact of dose, patient adherence, and drug resistance. By including inputs from more sophisticated and realistic viral dynamic models, these epidemiology models aid decision making regarding managing influenza on a global scale.
Health economic models can then be used to determine the relative change in quality-adjusted life-years based on the predicted number of infected patients. These multi-scale models have the potential to help optimize influenza control, treatment, and care. Most of these questions would be difficult to answer via traditional clinical trials.
We anticipate that this ‘pharmacology to payer’ framework will be extended to inform decisions regarding managing existing and emerging pathogens on a global scale. This framework can be employed to justify drug costs to payers and also to determine the likely transmission patterns for infectious diseases, helping to optimize use of stockpiled national medical resources.
Future directions in modeling influenza virology and pharmacology
Over the past four decades, modeling and simulation has been used to increase influenza knowledge and advance antiviral drug development. Computational power and mathematical modeling methods continue to evolve. Thus, having a framework to link sophisticated models from different scientific disciplines will improve our ability to characterize the mechanisms governing individual and public health outcomes of influenza infection. By leveraging modeling and simulation to gain greater understanding of the influenza virus and strategies to combat it, we are developing critically important weapons for our infectious disease armamentarium.
Mark Lovern is executive director and Patrick Smith is chief scientific officer of Certara Strategic Consulting Services. Suzanne Minton is scientific communications manager at Certara.




