
Dual-channel UHPLC for simultaneous multi-parameter mAb characterization. Credit Courtesy of Thermo Fisher Scientific.
The volume of biopharmaceuticals reaching the clinic continues to increase, and these products now account for an estimated 40 percent of the overall pharmaceutical pipeline, according to a recent report from industry consultants BioPlan Associates. Monoclonal antibodies (mAbs) are currently amongst the largest of the different classes of biotherapeutic products, with their success driven in large part by their strong efficacy and excellent target specificity, as well as ongoing improvements in production costs.
Like other types of biotherapeutics, mAbs are large and structurally complex molecules that are generated through advanced production processes. However, given their complexity and robust quality, control measures are required that go beyond those applied in the manufacture of traditional small molecule therapeutics. To deliver products that are both safe and effective, the accurate characterization of mAbs is essential to detect the presence of post-translational modifications, aggregates and other impurities. Often, these impurities are structurally similar to the active drug, affecting product stability and activity, and potentially triggering adverse reactions in the patient.
As such, the identification, characterization and monitoring processes required by mAb production workflows can be highly demanding of the analytical techniques employed. The methods used must be capable of distinguishing between molecules of very similar size and mass, provide accurate identification and be sufficiently sensitive to detect analytes at trace levels. In many cases, specialist characterization techniques must be used that are focused towards a specific type of product. With quality control laboratories under continued pressure to process a large number of products while accelerating time to results, the workflows they adopt must be highly efficient and flexible, and also deliver reliable results.
The need for high-throughput, flexible mAb characterization
Ultra-high performance liquid chromatography (UHPLC) has established itself as a powerful tool for the characterization of biopharmaceutical products such as mAbs, thanks to the exceptional separation resolution, sensitivity and speed that gives it the edge over conventional HPLC techniques. Used in combination with optical (UV or fluorescence) detection, mass spectrometry (MS), or charged aerosol detection, UHPLC enables the collection of highly accurate and precise product characterization information.
However, one of the biggest challenges currently associated with UHPLC workflows is throughput. While UHPLC systems can analyze samples at speed, standard instrument setups permit the use of only one stationary phase at a time. This imposes severe limitations when monitoring multiple characteristics of mAbs as separate analytical runs must be performed sequentially, increasing the time and cost associated with analyses. While throughput may be improved by using multiple instruments, constraints on budgets and physical space often make this an unattractive option for many laboratories.
The advent of dual-channel UHPLC systems that incorporate a second channel for separate simultaneous analysis on a single instrument is easing throughput constraints. With two pumps and two detector systems, these instruments enable analysis using two different LC methods through two flow paths, allowing the performance of two separate experiments in parallel (Figure 1). This has clear benefits for improving productivity and efficiency, yielding more information, and reducing the cost per sample.
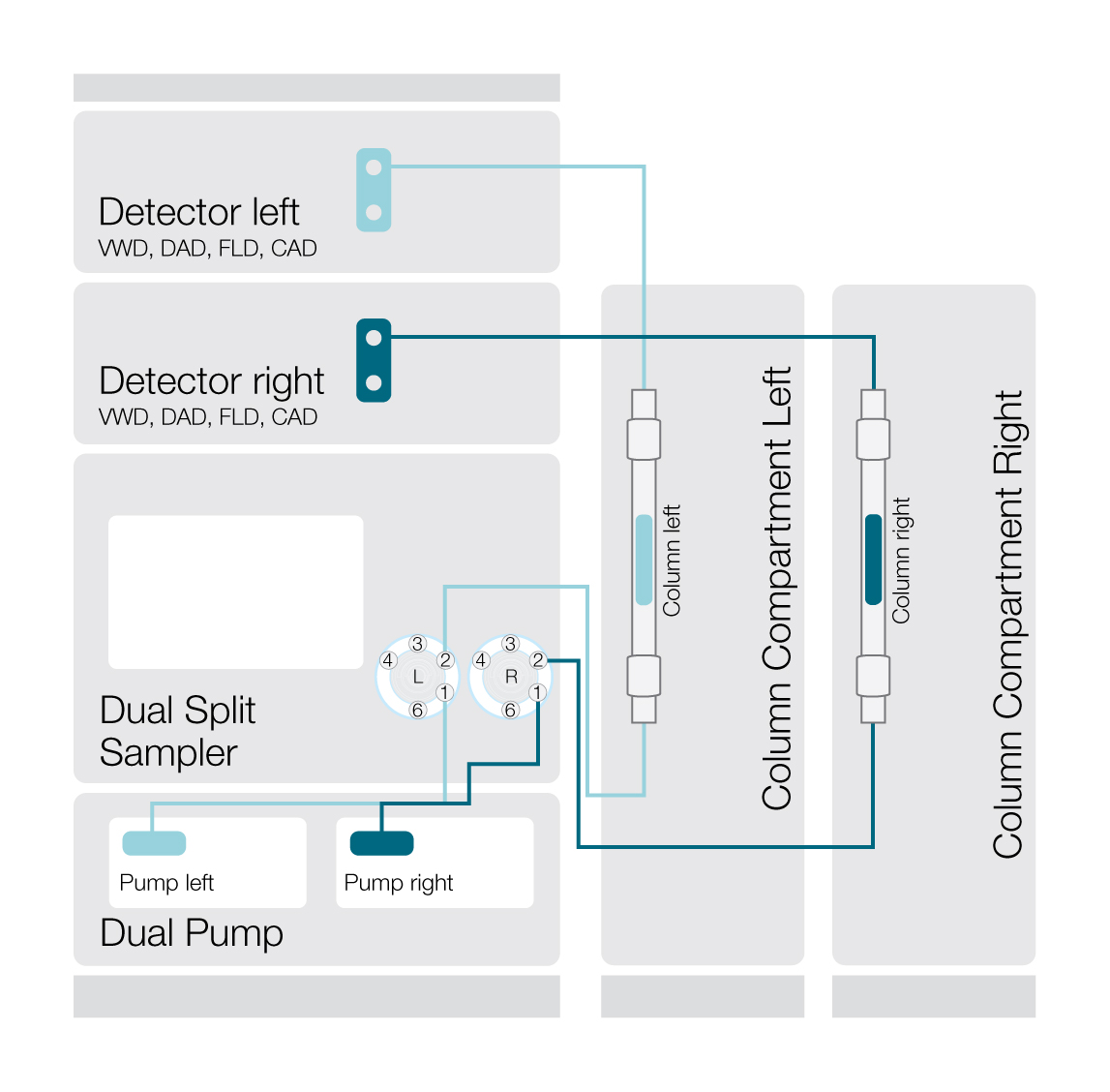
Figure 1. Dual-channel setup of the Thermo Scientific Vanquish Flex Duo system for dual LC
Simultaneous analysis of mAbs using two characterization techniques
To be effective in high-throughput bioanalysis workflows, dual-channel UHPLC systems must enable efficient output and method adaptability without compromising data quality or reproducibility. The exceptional performance and flexibility of modern dual-channel UHPLC systems was demonstrated in a characterization study of two mAbs, bevacizumab and infliximab, using the Thermo Scientific Vanquish Flex Duo UHPLC system for dual LC. In this study, the analytical performance, flexibility and high-throughput capabilities of a dual-channel UHPLC for simultaneous multi-parameter mAb characterization were assessed.
Each mAb was analyzed by two orthogonal LC methods simultaneously using the same instrument. Bevacizumab was characterized by size exclusion chromatography (SEC) aggregate analysis on one channel and strong cation exchange (SCX) chromatography charge variant analysis on the second channel. Infliximab was characterized by SCX charge variant analysis on the first channel and RP chromatography on the second.
The chromatography techniques applied in this study are highly valuable for mAb characterization. SEC aggregate analysis is commonly used to quantify the extent of protein denaturation that may have occurred during production or storage. Charge variant analysis by SCX chromatography is used to resolve molecules with different charge states that may have been formed by sequence truncations or glycan structure modifications. Reversed phase (RP) chromatography is often used to confirm the structure of intact mAbs. Each LC method employed distinct elution solvents and instrument parameters, including different LC gradients or isocratic conditions, data collection wavelengths, and total run times. Ten technical replicates were used in both sets of experiments.
High-throughput analysis without compromising data quality
Key chromatography parameters for the charge variant analysis and aggregate analysis of bevacizumab are presented in Table 1. The same parameters for the charge variant analysis and RP intact analysis of infliximab are shown in Table 2. Both sets of data highlight the excellent reproducibility of the dual-channel UHPLC system. The low percentage relative standard deviation (RSD) calculated for each of the parameters highlights the strong run-to-run consistency offered by the system.
Table 1. Performance of charge variant analysis and aggregate analysis of bevacizumab
|
|
|
Retention Time (min) |
Relative Peak Area (%) |
Peak Width at 50% Height (min) |
Asymmetry (EP) |
|
SCX Analysis of Bevacizumab (n=10) |
Average
|
12.515 |
85.01 |
0.302 |
1.81 |
|
% RSD
|
0.16 |
0.30 |
5.00 |
1.48 |
|
|
SEC Analysis of Bevacizumab (n=10) |
Average
|
9.554 |
96.57 |
0.259 |
1.08 |
|
% RSD
|
0.03 |
0.29 |
0.25 |
1.00 |
Table 2. Performance of charge variant analysis and reversed phase intact analysis of infliximab
|
|
|
Retention Time (min) |
Relative Peak Area (%) |
Peak Width at 50% Height (min) |
Asymmetry (EP) |
|
SCX Analysis of Infliximab (n=10) |
Average
|
11.856 |
54.08 |
0.293 |
1.03 |
|
% RSD
|
0.15 |
0.57 |
1.92 |
1.66 |
|
|
RP Analysis of Infliximab (n=10) |
Average
|
5.477 |
100.00 |
0.0746 |
1.34 |
|
% RSD
|
0.072 |
0.00 |
1.92 |
1.57 |
Importantly, using dual-channel UHPLC for the simultaneous characterization of the two mAbs significantly reduced the total time required for the analyses compared to sequential experimental setup (Figure 2). The dual-channel system not only shortened data acquisition time, but also eliminated the need to changeover system conditions between experiments. The efficiency savings offered by dual-channel systems provide considerable benefits for laboratories that must use multiple validated chromatography methods.
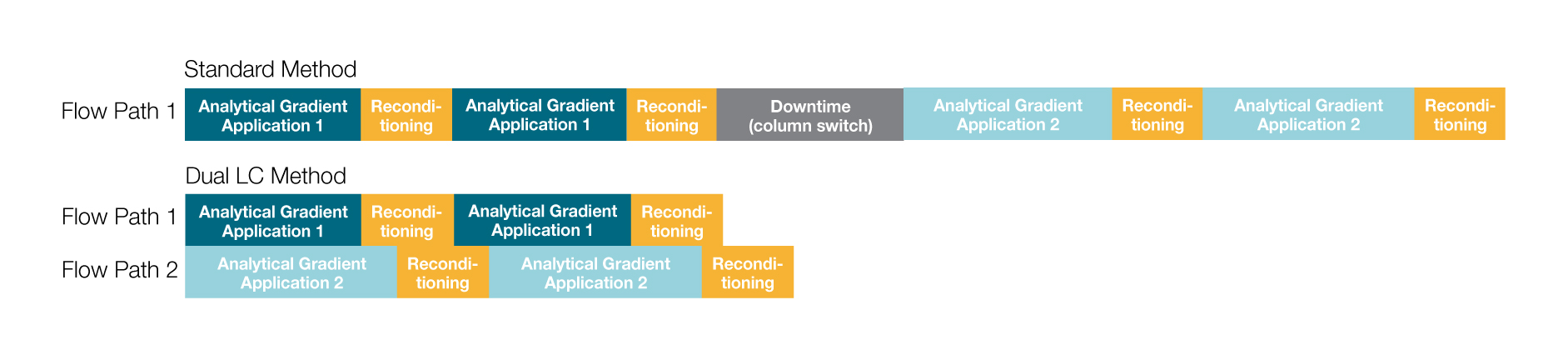
Figure 2. Time savings gained by using dual-channel setup
Conclusion
Rapid growth in the number of structurally complex biopharmaceutical products such as mAbs reaching the market means that developers and manufacturers are under continued pressure to perform in-depth drug characterization on a large scale. Advanced dual-channel UHPLC systems are helping laboratories balance the need to generate reliable characterization data with the demand for efficient high-throughput workflows. The mAb characterization studies presented here highlight the utility of dual-channel UHPLC systems and the quality and consistency of the data they produce. These innovative systems are helping scientists achieve more from their workflows, without compromising data quality.
Sara Carillo, PhD, works at the National Institute for Bioprocessing Research and Training in collaboration with Thermo Fisher Scientific for the development on new analytical approaches in biopharma. She has also worked to understand the effects of extractable and leachable from single-use bioreactors on CHO cells N-glycome.
Jonathan Bones, PhD, is the principal investigator of the National Institute for Bioprocessing Research and Training Characterization and Comparability Laboratory and an Associate Professor at University College Dublin. His primary research is focused on the development and application of liquid phase separations and mass spectrometry for the analysis of complex biological systems.



