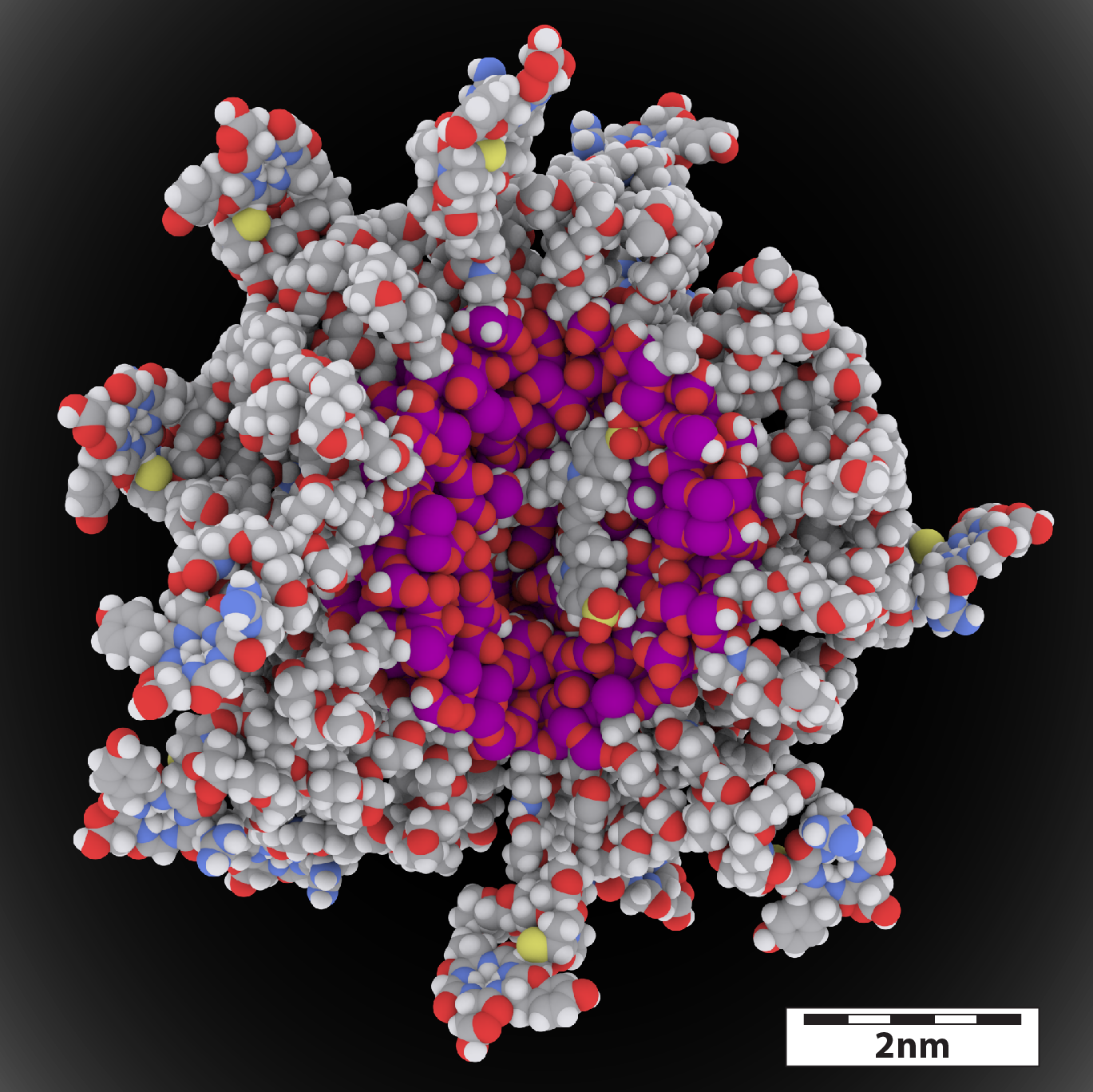
Figure. A molecular rendering of C dot.
Clinicians have often pondered if cancer cells could be selectively targeted, and how payloads ranging from fluorescent dyes to oncology drugs could be accurately delivered to these cells, and then safely cleared through the kidneys. Now, researchers at Memorial Sloan Kettering Cancer Center, and Nanotechnology in the College of Engineering at Cornell University have found the answer in the form of a new class of ultra-small nanoparticles that are showing significant potential for cancer diagnosis and treatment, and positive results in pre-clinical and clinical trials.
Researchers are finding that the use of new ultra-small nanoparticles called C-dots allows improved visualization in Positron Emission Tomography (PET) scans for diagnosis and real-time image-guided intraoperative mapping of nodal metastases to aid in precise surgical excision, currently in Phase II human clinical trials.
These ultra-small particles can also serve as a precision therapeutic solution, targeting and penetrating cancer cells to deliver a range of cancer-killing drugs, with the promise of unused particles and the drugs they carry safely leaving the body through the renal system.
The architecture of the C-dot is an ultra-small silica nanoparticle with a diameter of less than 10 nanometers, first innovated at Cornell University by Ulrich Wiesner, the Spencer T. Olin Professor of Engineering. Silica, also known as silicon dioxide (SiO2), is a widely existing inorganic composition which can be found in many places, e.g. plants, cosmetics, medicine and food such as bread crust.
Multiple near infrared (near-IR) fluorescent dye molecules can be covalently encapsulated inside the silica particle, and the fluorescence brightness of these molecules is further enhanced by the rigid surrounding silica matrix. As a result, the overall fluorescence brightness of C-dots can be one order of magnitude higher than that of dyes alone, delivering new capabilities to surgeons. Meanwhile, the surface of C-dots is covalently covered with polymer chains to increase bio-compatibility. Due to the high versatility of the synthesis chemistry of C-dots, different functional ligands can be selectively attached to the end of some of the polymer chains on the C-dot surface to endow the particles with a variety of functionalities for different applications, including, but not limited to cancer targeting, radio-isotopes chelating, and small molecule drug delivery.
Importantly, the ultra-small hydrodynamic size of C-dots enable them to be efficiently cleared from the body through the renal system. The hope of this ‘Target or Clear’ characteristic of C-dots is to improve dose-limiting toxicity associated with current cancer drugs which achieving improved therapeutic index.
Preclinical studies using a well-established spontaneous melanoma miniswine model, have demonstrated the targeting ability of C-dots by revealing specific accumulation of cancer targeting C-Dots in sentinel lymph node (SLN) metastases. The high cancer targeting efficacy also renders C-Dots a promising drug delivery platform to transport a range of therapeutic options specifically to tumors.
Led by Prof. Michelle Bradbury at Memorial Sloan Kettering Cancer Center, researchers have successfully helped to co-develop and translate C-Dots from the laboratory to the clinic. C-Dots are the first inorganic optically-active platform of their kind to obtain authorization from FDA as investigational new drug (IND) for clinical use. In the Phase 1 first human clinical trial of C dots, near-IR fluorescent dyes were encapsulated inside the silica while cancer-targeting radioiodinated peptides were bound to the polymers on the C-Dot surface, allowing the C-Dots to be traced using both optical imaging and PET. Three days after intravenous injection into melanoma patients, the majority of C-Dots were excreted in urine. At the same time, lesion uptake by C-Dots was clearly observed in some patients, while no accumulation of C-Dots in healthy organs was detected. This pharmacokinetic and clearance profile of C-Dots, i.e. Target or Clear has demonstrated safety in human trials, as well as the potential of the platform in cancer detection and treatment.
Based on success in the first human trials, the MSKCC-Cornell Center for Translation of Cancer Nanomedicines was launched, bringing together scientists, physicians, engineers, and Elucida Oncology, Inc. to co-develop, translate, and commercialize new cancer care applications based on C-Dot nanotechnology.
Additionally, as part of a Phase 2 clinical trial, the high fluorescent brightness of C-Dots is being exploited alongside real-time optical imaging devices to enable surgeons to visualize cancerous tissues in-real time in order to facilitate a more precise surgical excision.
Based on partnerships with Memorial Sloan Kettering Cancer Center and Nanotechnology in the College of Engineering at Cornell University, this technology is currently being commercialized by Elucida Oncology, and offers great promise for the future of treatment of a range of cancers.
The Elucida Oncology name is a registered trademark of Elucida Oncology, Inc. Target or Clear is a Trademark of Elucida Oncology, Inc.




