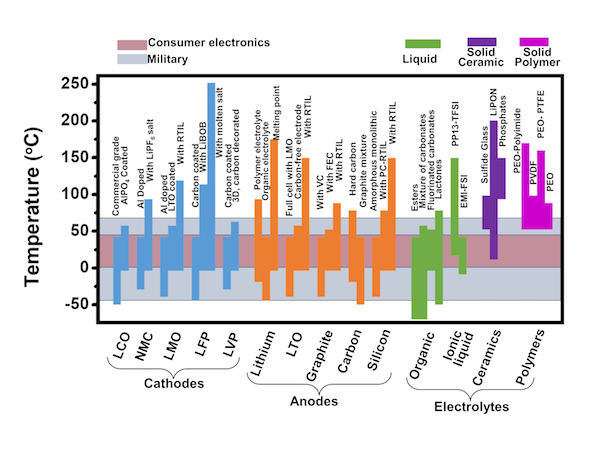
A map created by materials scientists at Rice University will help labs develop lithium-ion batteries for extreme environments. Click on the image for a larger version. Courtesy of the Ajayan Group
Cell phones may soon be able to handle the extreme heat of a beach day or frigid cold of a day at the ski slopes.
Researchers from Rice University have developed a guide on how to create lithium-ion batteries used in cell phones and electric cars that are more adaptable for challenging conditions.
The research team reviewed and analyzed their own past work and the work of peers involving lithium-ion batteries and the problems that are persistent.
“We searched hard to find one paper that talks about all the problems at the same time and what all the individual components experience at extreme temperatures, and we couldn’t find one,” said Hemtej Gullapalli, a postdoctoral researcher at Rice and co-author of the paper, said in a statement. “So we believe this is a good opportunity to survey the field.”
Current batteries are designed to operate near room temperature and within a narrow temperature range. To create the guide, researchers focused on how a battery responded to temperatures between 76 and 302 degrees Fahrenheit to simulate some of the extreme temperatures an electric car battery may face.
“People have not looked that studiously at temperature constraints,” Gullapalli said. “Most research involving batteries and temperatures involve management systems: For instance, if a phone is used in cold temperatures, they slow it down a little bit to preserve the battery.
“But we found in our review that the perspective is changing slightly,” he added. “To make batteries that work from low to high temperatures, scientists have to take the materials perspective to see what temperature is specifically doing to the materials.”
Electrochemical batteries—which contain a negative anode, a positive cathode and a conducting electrolyte that allow electrons to move from one side to the other while either charging or draining—may offer opportunities for a better battery in extreme conditions.
“People have done amazing work,” Gullapalli said. “They’ve touched almost the whole periodic table and all the permutations and combinations have been tested.
“Now we’re into the engineering phase where we know the materials’ limitations and we are trying to break down the barriers.”
The research team has come up with both standard and promising new materials in commercial batteries, which could help future researchers pick a required combination that fits their needs.
“Building an ideal or a close-to-ideal system requires a thorough understanding of the subtle mechanisms and replacing each delinquent component with a suitable alternative,” Rice materials scientist Pulickel Ajayan, said in a statement. “A trivial component at ambient conditions can change the whole electrochemistry when exposed to high temperatures.”
The study was published in Nature Energy.




