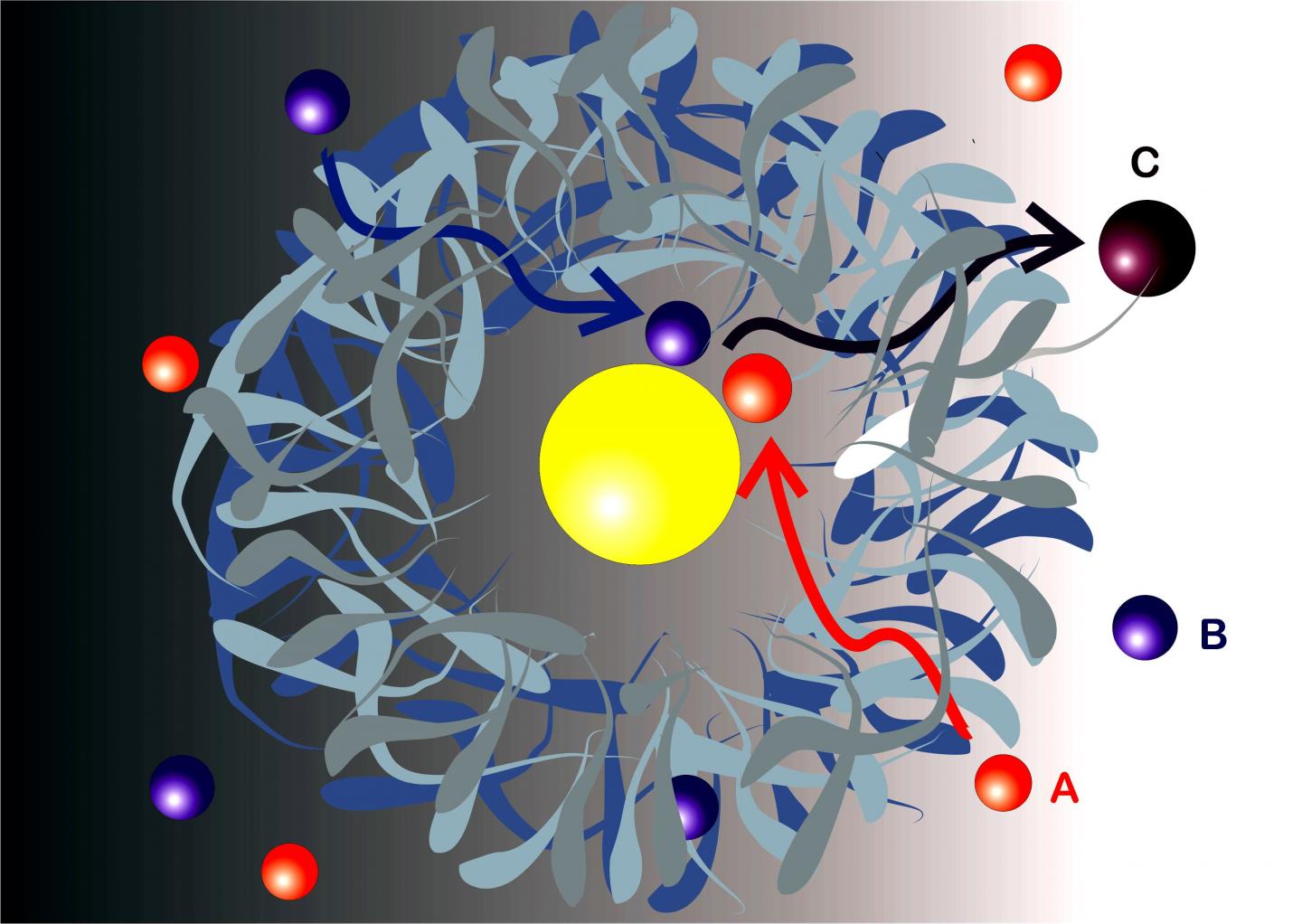
Reactants A and B diffuse through the shell and react to product C at the catalytically active nanoparticle (yellow) inside.
Nanoreactors are tiny systems which facilitate specific chemical reactions, as a catalyst does. Many are found in biological systems, such as certain proteins. But chemists are also able to synthesise artificial nanoreactors to control chemical reactions. An important class of these nanoreactors has a “yolk & shell” architecture like an egg: a catalytically active metallic nanoparticle is surrounded by a shell consisting of a polymeric network. These kinds of nanoreactors can create isolated environments for specific reactions and restrict them to the tiny space inside the shell.
Mathematical description delivers new insights
“We have now mathematically described for the first time how two different molecules are transported to react within nanoreactors. The new model shows clearly what factors favour a given reaction”, says Dr. Rafael Roa. Roa is first author of the publication in ACS Catalysis and a postdoc in the group headed by Prof. Joe Dzubiella at the HZB Institute for Soft Matter and Functional Materials.
What matters most?
Some of the results come as a surprise: contrary to expectations, the reaction rate is not so much limited by the concentration of the molecules in solution, but decisively by the permeability of the nanoreactor’s shell. “This is extremely interesting since chemists today can often fine tune or even switch the permeability of these shells to specific molecules via variations in temperature or other parameters”, explains co-author Dr. Won Kyu Kim.
Photo-activation taken into account
The new model is a big step forward from the older theory done many decades earlier that could handle only one molecule. “Our model is applicable to research on energy materials, and it can even take into account photo-activation of one of the molecules at the shell by sunlight”, Dzubiella states. He has achieved with this work one of the goals of his European Research Council (ERC) Consolidator Grant (2015-2020).
Predictions will be put to test
Dzubiella’s Soft Matter Theory group collaborates with HZB chemist Prof. Yan Lu, an acknowledged expert in synthetic nanoreactors. They are eager to test their theoretical predictions on real systems. “We’re now able to better understand what happens, and we expect to predict how the catalytic effects of these types of nanoreactors can be controlled – through feedback loops, for instance, which will stop or start the reaction at will.”