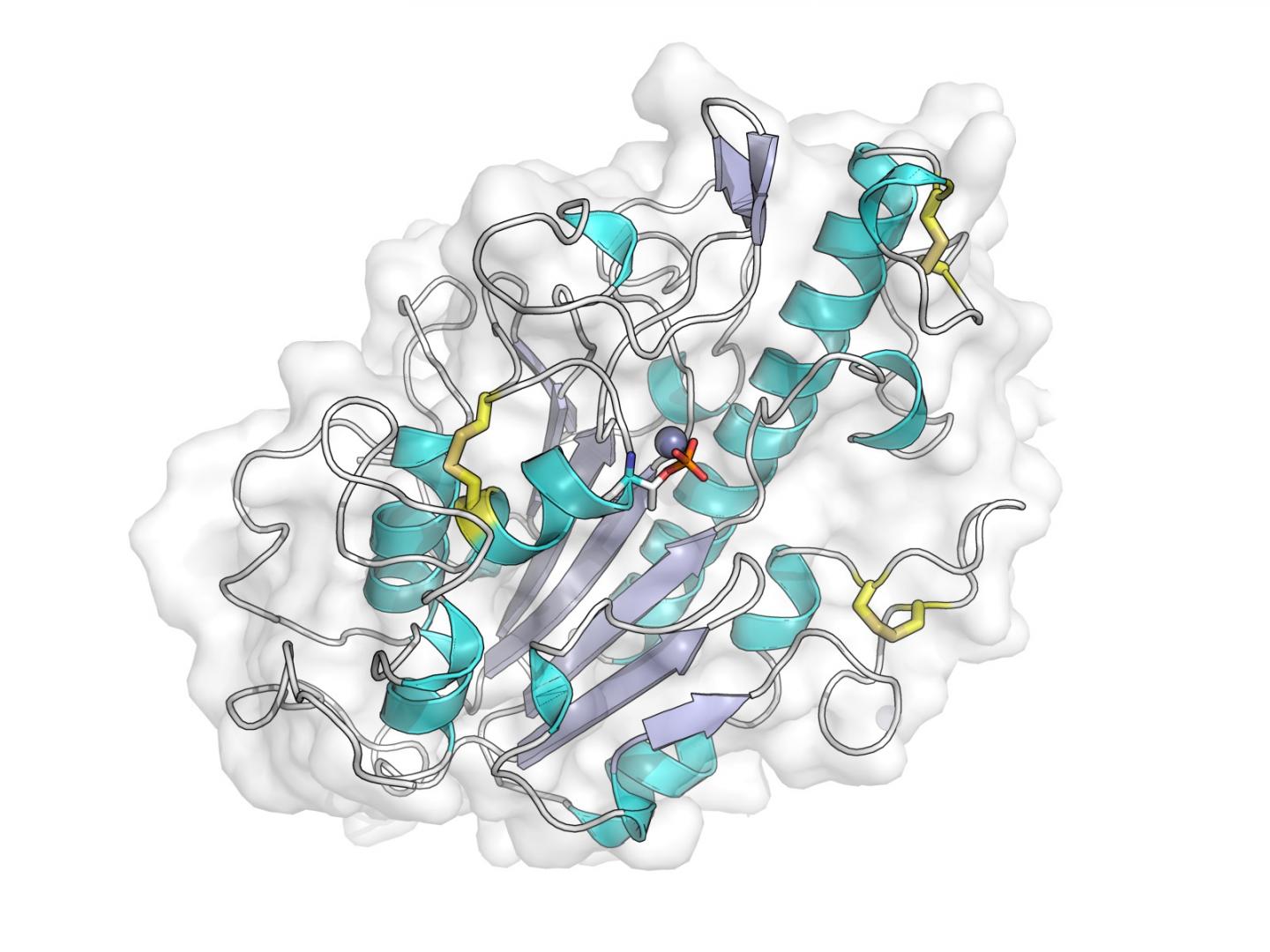
X-ray crystal structure of the catalytic domain of the MCR-1 protein. Source: Dr. Phil Hinchliffe
An international research team, led by the University of Bristol, has provided the first clues to understand how the mcr-1 gene protects bacteria from colistin – a ‘last resort’ antibiotic used to treat life-threatening bacterial infections that do not respond to other treatment options.
Last year, members of the team, led by Dr Jim Spencer from the School of Cellular and Molecular Medicine, in collaboration with colleagues from Oxford, Cardiff, Diamond Light Source, Thailand and China, identified mcr-1 as the first colistin-resistance gene that could be passed between bacteria, enabling resistance to spread rapidly within a bacterial population.
Since then, the mcr-1 gene has been detected in common bacteria, such as E. coli, in China, the United States and across Europe first in farm animals and recently – worryingly – in human patients. The spread of mcr-1 has been linked to agricultural use of colistin, indicating that transmission between animals and humans may take place. In response to these findings the Chinese government has now banned use of colistin in animal feed.
Colistin acts by binding to, and disrupting, the outer surface of bacteria. Bacteria carrying the mcr-1 gene make a protein that modifies the bacterial surface to reduce colistin binding, making the organism resistant.
In their work the team used X-rays produced at Diamond’s crystallography beamlines to generate detailed pictures of the portion of this protein responsible for this modification, and with this information identified key features that are necessary for it to function.
They also constructed computer models of the chemical reaction that leads to resistance.
This provides the first clues as to how mcr-1 acts within the bacterial cell, as well as information essential to efforts to identify ways of blocking MCR-1 function that could restore the activity of colistin against bacteria carrying mcr-1.
This work was funded by the UK Medical Research (MRC), Biotechnology and Biological Sciences (BBSRC) and Engineering and Physical Sciences (EPSRC) Research Councils, the Royal Society of Chemistry and government agencies from China and Thailand. Essential to the study was the involvement of researchers from multiple disciplines, including medical and veterinary microbiologists and organic, inorganic and computational chemists.
In particular BristolBridge, an EPSRC-funded research initiative at the University of Bristol that aims to engage researchers in the physical sciences and engineering with the problem of antimicrobial resistance, helped bring to the project expertise in inorganic and computational chemistry which proved invaluable in understanding the experimental results.
Professor Adrian Mulholland, co-author of the study and Principal Investigator for the BristolBridge initiative, based in the School of Chemistry, said: “The importance of understanding colistin resistance can hardly be overstated: it is rapidly emerging threat to public health.
“Our results illuminate the structural and (for the first time) mechanistic basis of transferable colistin resistance conferred by mcr-1, thanks to the combination of biological, chemical and computational expertise brought to bear on this project.
“We are confident that our findings will drive efforts to understand mcr-1-mediated resistance and ultimately help identify routes towards overcoming MCR-1 activity in harmful bacteria.”




