The highly pathogenic Staphylococcus aureus bacteria is one of the five most common causes of hospital-acquired infections. In the U.S. alone, approximately 500,000 patients at hospitals contract a staph infection. It is the bacteria responsible for MRSA, for which there is no vaccine.
But all that could change, thanks to groundbreaking findings published in Science by a Technion-Israel Institute of Technology team led by Assistant Professor Meytal Landau of the Faculty of Biology. The researchers discovered, for the first time, unique amyloid fibrils through which the pathogenic and highly drug resistant Staphylococcus aureus bacterium attacks the human cells and immune system. The research could advance the discovery of antibiotics with a novel mechanism of action that will attack key bacterial toxins.
The researchers discovered “ammunition” that assists the infectious bacterium: a novel form of an amyloid fibril whose three-dimensional structure was determined at atomic resolution, revealing the first-of-its-kind structure of this toxic fibril. Amyloids, which are proteins notoriously known for their association with neuro-degenerative diseases such as Alzheimer’s and Parkinson’s, form a network of protein fibrils — somewhat similar to a spider web — characterized by an orderly and extremely stable structure. This stability enables the proteins to withstand extreme conditions in which ordinary proteins do not survive.
One of the best-known examples of this is “Mad Cow” disease, which broke out in England in 1986. According to Landau, “This disease surprised the scientific community because its cause was not a virus, nor a bacterium, but a protein called Prion, possessing an amyloid-like structure. It then became clear that a protein can be transmissible, and due to its stability, it infected human beings who consumed the contaminated beef — meaning, the protein did not break down in the stages of meat processing, cooking and digestion.”
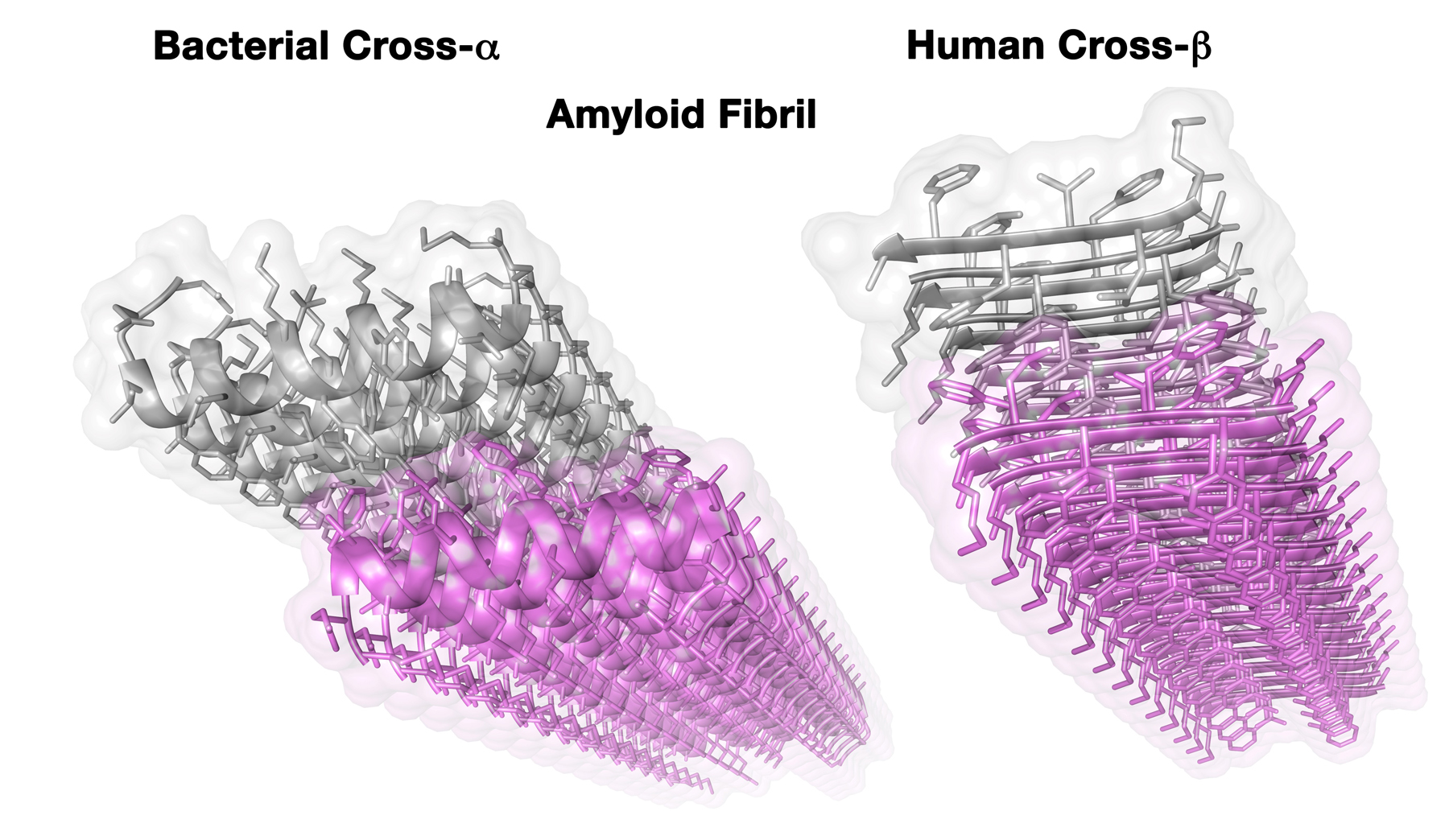
Crystal structures of disease-associated human amyloid fibril (right) and novel bacterial amyloid fibril (left). Three-dimensional structures of the fibrils were obtained using x-ray microcrystallography – a method that provides information at the single-atom level. The image depicts the long fibrils, which can theoretically be extended on both sides. The fibril is composed of two adjacent “surfaces” (purple and gray), called sheets, which mate via strong chemical interactions. Each sheet is built from self-association of peptides, horizontally running along the sheet. While the overall shape of mating sheets is similar among the human and bacterial fibrils, the basic units of the fibril (individual peptides) assume a completely different form. In the canonical amyloid structure observed in human diseases (right), the peptides form extended ribbons (called beta-strands). In the novel bacterial fibril (left), the peptides are spiral like telephone cords (called alpha-helices).
The “Mad Cow” prion, like all amyloids discovered so far, belongs to a group possessing what is called a cross-ß structure. In the present study, an amyloid of a completely new structure was discovered, which was named cross-α.
“At some point we knew that we had found something unique, but only after several trips to cyclic particle accelerators (Synchrotrons) in Grenoble and Chicago were we successful in verifying its being a new type of amyloid,” says Landau. “Much more work was required before we could publish our findings, but from the very first moment, it was clear to us that what we had was a paradigm shift.”
She estimates that the new discovery will lead to the development of antibiotics with a new action mechanism. Such drugs will inhibit the amyloid formation thereby neutralizing one of the important “weapons” in the arsenal of this pathogenic bacterium. In her opinion, since this antibiotic is not aimed at killing the bacterium but only reducing its toxicity to humans, it will not lead to a rapid development of bacterial resistance towards it.
“Resistance to antibiotics develops in bacteria due to evolutionary pressure — natural selection leads to the growth of bacteria which antibiotics are unable to kill,” she says. “If we reduce the pressure on the bacterium and don’t kill it but rather prevent its pathogenic aspects, the resistance will probably not rush to develop.”
Looking towards the future, Landau clarifies that “in academia itself, it’s difficult to carry out a full process of drug development due to the prohibitive cost entailed. Nevertheless, we can provide drug developers with scientific knowledge that will accelerate and lower the costs of the process. The present discovery is a stepping-stone in that respect. Now our challenge is to find the substance that will impede the cross-α fibril, thereby ‘disarming’ the bacterium.”
“From the scientific standpoint, there is an important lesson here — thinking out of the box opens new doors,” she continues. “In this specific case, extending the repertoire of amyloids. Deciphering new structures of amyloids might lead to new insights regarding mechanisms of neurodegenerative diseases. It might also lead to the discovery of ‘good’ amyloids that take part in an organism’s natural protection against infections. Such research could lead to the development of novel means of protection against the toxins found in bacteria and fungi.”
The research was conducted by members of the Landau lab, including Einav Tayeb-Fligelman, Orly Tabachnikov, Asher Moshe, and Orit Goldshmidt-Tran, with the assistance of Michael Sawaya from the University of California Los Angeles, and of Nicolas Coquelle and Jacques-Philippe Colletier from Université Grenoble.
Source: American Technion Society




