The fossil fuel fight goes on for USC scientists as they develop a new method for creating reversible hydrogen storage based on methanol, with no carbon emissions, in the last major paper co-authored by USC’s first Nobel laureate, the late George Olah. Credit: Sayan Kar
A new renewable energy source might come from the world’s simplest alcohol.
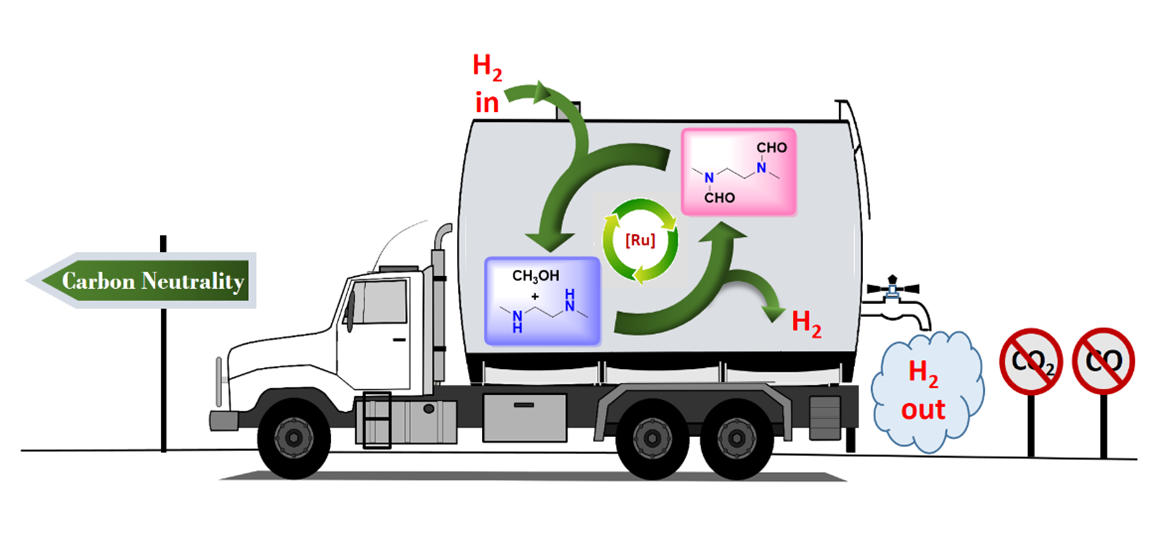
A team of researchers from the University of Southern California, led by senior author G. K. Surya Prakash and 1994 Nobel Prize winner George Olah, have created a carbon-neutral method to produce and store hydrogen from methanol, without concurrent production of either carbon monoxide or carbon dioxide.
The researchers were able to trap the hydrogen in organic derivatives of ammonia called amines.
The process to extract hydrogen from methanol, called the methanol reformer, traditionally produced carbon monoxide and carbon dioxide as part of the extraction process. However, because carbon dioxide is considered a greenhouse gas that causes global warming and ocean acidification, making a push to find a way to extract hydrogen without producing greenhouse gas has become important.
According to the study, by coupling methanol and 1,2-diamine, the reaction production of N-formamide and N, N′-diformamide, are hydrogenated back to the free amine and methanol by a simple hydrogen pressure swing.
“Thus, an efficient one-pot hydrogen carrier system has been developed,” the study states. “The H2 generating step can be termed as ‘amine reforming of methanol’ in analogy to the traditional steam reforming. It acts as a clean source of hydrogen without concurrent production of CO2 [unlike steam reforming] or CO [by complete methanol dehydrogenation].
“Therefore, a carbon neutral cycle is essentially achieved where no carbon capture is necessary as the carbon is trapped in the form of formamide [or urea in the case of primary amine]. In theory, a hydrogen storage capacity as high as 6.6 weight percentage is achievable.”
Methanol, which can be produced with only water, carbon dioxide and energy, stores half the energy of traditional petroleum-based gasoline while also burning cleaner with no soot, particulates or other residue and burning half as bright.
It naturally occurs in small amounts in the Earth’s atmosphere, but there are large clouds of methanol floating in the star-forming regions of space.
The combustion of methanol also produces less deleterious greenhouse gases of nitrogen oxides, according to the U.S. Environmental Protection Agency.
Methanol, which is sometimes called wood alcohol, biodegrades quickly and is traditionally produced from natural gas. it can be corrosive to older automobile tubing and casing, but much less to newer generations of vehicles. While it is more efficient to replace gasoline or diesel with methanol, the alcohol also provides less miles to the gallon due to its lower energy density.
The research team first began refining “The Methanol Economy” about 20 years ago with the goal of developing renewable sources of energy, led by methanol, that could mitigate the problems caused by carbon emissions, as well as the U.S. dependence on other countries for oil.
Since then the need to offset crude oil consumption has grown to where global consumption is expected to be about 100 million barrels by 2018.
The study was published in The Journal of the American Chemical Society. It represents Olah’s last major paper as he passed away on March 8, 2017.




