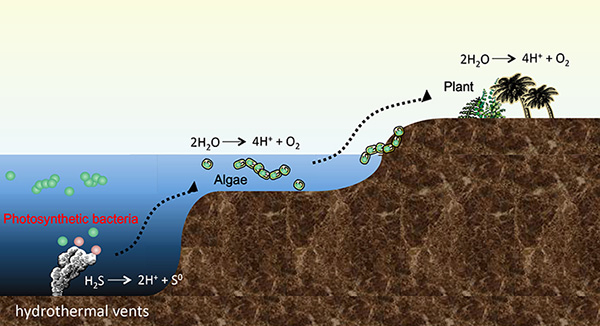
Photosynthetic bacteria that were born in the deep sea hydrothermal vents have since evolved into algae, land plants. They were born for the first time on the Earth, it is thought that hydrogen sulfide (H2S) was used instead of water (H2O) as the electron source, that was their one or only one energy source. Credit: Tokyo Institute of Technology
Scientists in Japan and the U.S. have discovered a sulfide-responsive protein that sheds light on the electron donation processes in the early evolution of photosynthesis.
Purple bacteria, including Rhodobacter capsulatus, has the ability to switch between energy sources and different electron donors depending on their surrounding environment, which differs from many micro-algae and plants that only use water as an electron donor to drive photosynthesis.
The purple bacteria must carefully control the synthesis of electron transfer proteins in response to changing conditions, but the precise mechanisms used by R. capsulatus to sense and make use of hydrogen sulfide is unclear.
A team led by Takayuki Shimizu and Shinji Masuda at the Tokyo Institute of Technology, in collaboration with David P. Giedroc and Carl E. Bauer at Indiana University and researchers across Japan and the USA, have uncovered and characterized a sulfide-responsive protein or transcriptional repressor called SqrR.
The team examined protein and gene responses in the bacteria and identified SqrR, which regulates around 45 percent of the genes responsible for sulfide-dependent photosynthesis in R. capsulatus.
According to the study, sulfide was used as an electron donor early in the evolution of photosynthesis, with many extant photosynthetic bacteria still capable of using sulfur compounds such as hydrogen sulfide (H2S) as a photosynthetic electron donor.
The research team discovered that when sulfides in the surrounding environment increase, SqrR responds by binding to the sulfide molecules, thereby repressing photosynthetic electron transfer so that the bacterium can survive sulfide stress.
This helps the protein maintain sulfide homeostasis in rapidly-changing environments.
According to the study, SqrR has three cysteine residues, two of which, C41 and C107, are conserved in SqrR homologs from other bacteria.
Further analysis with liquid chromatography coupled with an electrospray-interface tandem-mass spectrometer, reveals that SqrR forms an intramolecular tetrasulfide bond between c41 and c107 when incubated with the sulfur donor glutathione persulfide.
SqrR is oxidized in sulfide-stressed cells and tetrasulfide—cross-linked SqrR binds more weakly to a target promoter relative to unmodified SqrR
This discovery will allow scientists to examine photosynthesis in more depth and determine how bacteria have evolved to survive in different environments, while also having applications in synthetic biology.
Purple bacteria have been used by scientists to investigate the fundamental process of photosynthesis because they have adapted over millennia to respond rapidly to and survive multiple environmental stressors.
The bacteria has the ability to use different electron donors including hydrogen gas, methane and hydrogen sulfide and switch between different radiation sources.
The study was published in the Proceedings of the National Academy of Sciences of the United States of America.




