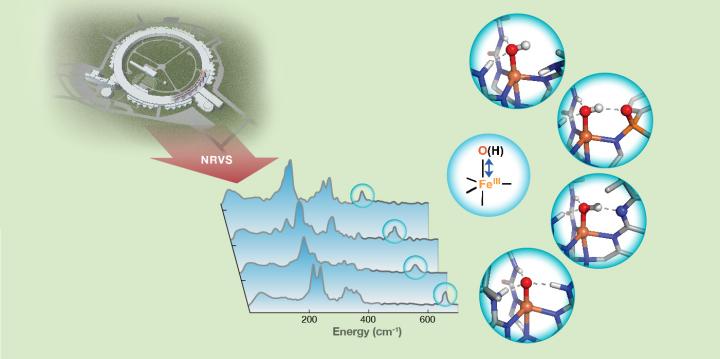
Hydrogen bond strength to iron(III)-oxido/hydroxido (FeIII-O/OH) units in nonheme iron complexes is revealed by FeIII-O/OH stretching vibrations detected with 57Fe nuclear resonance vibrational spectroscopy (NRVS). Credit: Carnegie Mellon University
Researchers have developed a new way to probe hydrogen bonds that could yield better catalysts for a number of applications in chemistry and biology.
A Carnegie Mellon University research team has found a way to probe hydrogen bonds that modulate the chemical reactivity of enzymes, catalysts and biomimetic complexes using nuclear resonance vibrational spectroscopy (NRVS).
Hydrogen bonds are responsible for several interactions in biology and chemistry including the chemically important properties of water, and to stabilize the structures of proteins and nucleic acids, including those found in DNA and RNA. Hydrogen bonds also contribute to the structure of natural and synthetic polymers.
Hydrogen bonds also play a crucial role in tuning the reactivity of the metal centers of metalloenzymes and metal containing catalysts.
Despite knowing how important hydrogen bonds are, researchers have not yet done extensive research to experimentally demonstrate how systematic changes to hydrogen bonds within the secondary coordination sphere—where molecules found in the vicinity of metal centers that do not have direct bonding interactions with the center—influence catalytic activity.
Enzymes or synthetic catalysts spur on a chain of chemical reactions in catalysis, which produce a number of intermediate structures or species. A better understanding of these structures and their chemical properties would enable a better understanding of the entire reaction.
“Thoroughly understanding the chemical reactivity of the reactive intermediate is a key step to determining how to design highly efficient and selective catalysts for C-H functionalization,” Yisong Guo, assistant professor of chemistry at Carnegie Mellon and the study’s lead author, said in a statement. “In the case of dioxygen-activating enzymes, the key intermediates of catalysis are iron-oxo [Fe-O] and iron-hydroxo [Fe-OH] species, which are involved in important biological processes, such as DNA biosynthesis, DNA and RNA repair, post-translational modification of proteins, biosynthesis of antibiotics and degradation of toxic compounds.”
The researchers used 57Fe NRVS—which is a newly developed synchrotron radiation-based technique—to identify the vibrational frequency of Fe-O and Fe-OH units of synthetic complexes that interact with the secondary coordination sphere through hydrogen bonds.
Changes in the frequencies revealed crucial information about the bond strengths of the units and provided a further qualitative measure of hydrogen bond strength.
“This showed that NRVS is a sensitive technique to pick up very small changes in hydrogen bond strength, down to the changes of a single hydrogen bond,” Guo said. “This provides us with a new method to connect changes in bond strength of Fe-O and Fe-OH units to their chemical reactivity.”
According to Guo, the study is a proof-of-concept for using NRVS to probe hydrogen bonds. The researchers plan to continue using the NRVS method to study more iron-oxo and iron hydroxo species in both synthetic complexes and enzymes to produce more data to correlate chemical reactivity of these species with the changes of hydrogen bond interactions.
They hope with more information they could ultimately develop more efficient and effective catalysts.
The study was published in Angewandte Chemie.



