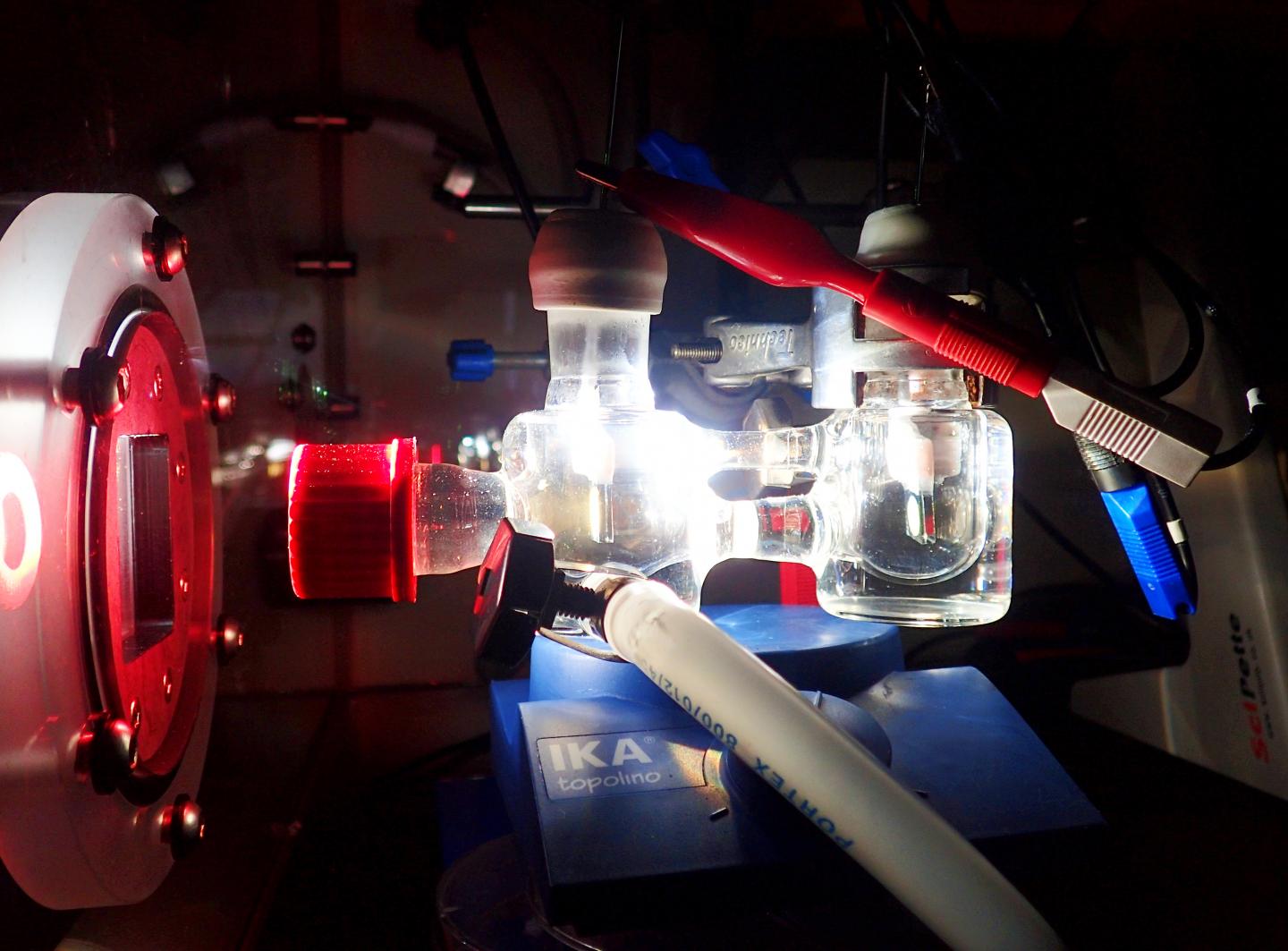
Experimental two-electrode setup showing the photoelectrochemical cell illuminated with simulated solar light. Credit: Katarzyna Sokól
New technology using semi-artificial photosynthesis could yield future renewable energy systems.
Researchers from St. John’s College and the University of Cambridge have discovered new ways to produce and store solar energy using semi-artificial photosynthesis, by combining biological components with man-made technologies to convert water into hydrogen and oxygen.
According to the study, semi-artificial photosynthesis combines the strengths of natural photosynthesis with synthetic chemistry and materials science to develop model systems that overcome nature’s limitations, including low-yielding metabolic pathways and non-complementary light absorption by photosystems I and II.
The research team are the first to successfully use the enzyme hydrogenase and photosystem II to develop semi-artificial photosynthesis driven strictly by solar power that absorb more solar light than natural photosynthesis.
“This work overcomes many difficult challenges associated with the integration of biological and organic components into inorganic materials for the assembly of semi-artificial devices and opens up a toolbox for developing future systems for solar energy conversion,” Erwin Reisner, PhD, Head of the Reisner Laboratory, a Fellow of St John’s College, University of Cambridge, and one of the paper’s authors, said in a statement.
While scientists have long used artificial photosynthesis, no one has been able to develop renewable energy production system that do not use catalysts that are neither toxic nor expensive.
“Natural photosynthesis is not efficient because it has evolved merely to survive so it makes the bare minimum amount of energy needed—around 1-2 per cent of what it could potentially convert and store,” Katarzyna Sokól, first author and PhD student at St John’s College, said in a statement.
However, the new technology developed at Cambridge is part of an emerging semi-artificial photosynthesis field, where researchers are seeking to overcome the limitations of fully artificial photosynthesis.
The researchers were able to increase the amount of energy produced and stored, as well as reactivate a process in algae that has been dormant for millennia.
“Hydrogenase is an enzyme present in algae that is capable of reducing protons into hydrogen,” Sokól said. “During evolution this process has been deactivated because it wasn’t necessary for survival but we successfully managed to bypass the inactivity to achieve the reaction we wanted—splitting water into hydrogen and oxygen.”
The researchers next plan to develop new model systems to convert solar energy.
“It’s exciting that we can selectively choose the processes we want, and achieve the reaction we want which is inaccessible in nature,” Sokól said. “This could be a great platform for developing solar technologies. The approach could be used to couple other reactions together to see what can be done, learn from these reactions and then build synthetic, more robust pieces of solar energy technology.”
The study was published in Nature Energy.




