Monoclonal antibodies are an invaluable component of drug discovery and diagnostic development pipelines. Today, however, it takes too long and costs too much to custom-generate effective antibodies. The pharma and biotech communities rely too heavily on these reagents to continue producing them via traditional methods.
Those methods, which include longtime standbys such as phage display and hybridomas, lead to an unacceptably broad range of affinity and narrow spectrum of specificity of antibodies for each antigen. Testing every antibody generated is expensive and time-consuming; therefore, a method of generating high-quality antibodies that reduces the number of antibodies required to be validated is essential.
More recently, scientists have adopted single-cell workflows for antibody production, with significant benefits for antibody quality. Plasma cells are the terminal differentiated B cells, containing the most mature antibody of terminal somatic mutagenesis. Their major function is synthesizing and secreting antibodies. A new method that builds on these single-cell advances incorporates plasma cells as a starting point to increase the likelihood of producing a monoclonal antibody with high affinity and specificity, while reducing the need to screen massive numbers of antibody candidates.
Common methods
Conventional approaches to antibody production have been used for decades. While most are still in use in labs around the world, some are destined for replacement in light of recently improved methods.
Phage and yeast display are antibody development mainstays. Because these methods do not involve immunizing animals and waiting for the development of an immune response, they can be faster than other protocols. But synthesizing V-gene libraries, the foundation for phage or yeast display, often necessitates screening diverse libraries of candidate antibodies. The search for an effective antibody among billions of options can be extremely costly, especially when maturation steps are needed to increase affinity.
Hybridomas are another well-worn path for producing antibodies. The process involves immunizing a mouse or other animal with the target antigen, followed by fusing its spleen cells with myeloma cells. The platform is reliable, but since any B cell can fuse with a myeloma cell, it produces a lot of no-antigen-specific antibodies and low-affinity antibodies from naïve or early-stage B cells. Furthermore, the low fusion rate (one in 105 cells) limits the diversity of antibodies generated from hybridoma technology. Another challenge with using hybridomas is turnaround time. With the immunization process and multiple rounds of limited dilution to stabilize the hybridoma, the entire production timeline can be as long as six months.
Single-cell techniques
A more recent method is single B cell culture and cloning. This method involves isolating single B cells (or a few B cells together) in a well and allowing them to divide for a couple of weeks, and then screening to find wells with immunoglobulin secretion. B cell culture and cloning methods bypass the bottleneck of low fusion rates seen with hybridomas, but like hybridomas, a high proportion of antibodies are relatively low affinity and large-scale screening is required.
Taking advantage of FACS (fluorescence-activated cell sorting) technology, individual memory cells have been used to develop HIV neutralization antibodies from blood cells of infected patients in a few academic laboratories at the frontier of fighting AIDS. These memory cells, like plasma cells, are a branch of rare B cells containing mature antibodies. Using them increases the likelihood that antibodies produced will have high affinity. Unfortunately, memory cell surface markers are not present in rabbit, limiting the utility of this approach.
A new single-cell-based platform has been developed in our labs. Instead of B cells or memory cells, single plasma cells are used for antibody development. Plasma cells have the benefit of containing 100 times or more antibody mRNA than other B cells, and harbor the antibodies at the end point of antigen-dependent somatic mutagenesis. Our method requires isolating the rare population of antigen-specific mature terminal plasma cells, which means that each antibody generated is far more likely to have high affinity and specificity. Compared to single B cell cloning, there are relatively fewer plasma cells needed for finding a high-affinity antibody, reducing the need for downstream screening. The process takes just two weeks. Importantly, it can be performed with rabbits, which have a larger antibody repertoire than mice and therefore can generate more diverse products.
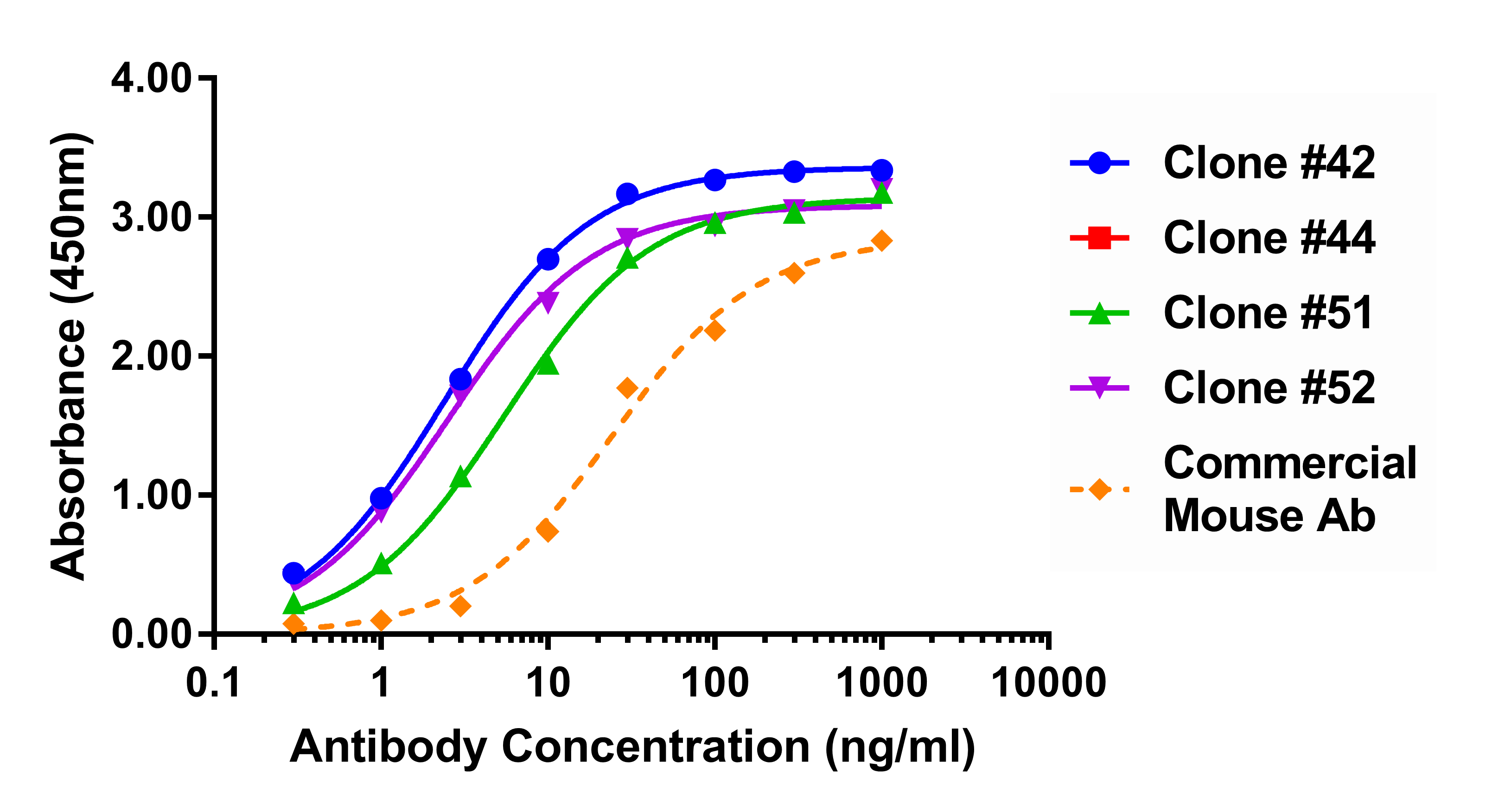
The single plasma cell method was used to generate a monoclonal antibody to human troponin I, with results outperforming a commercially available IVD anti-human troponin I antibody produced through a mouse hybridoma.
Implications
Scientists who need high-quality antibodies can advance their research by combining the benefits of a single-cell workflow, plasma cells containing mature antibodies, and production with rabbits to generate more diverse and higher-affinity antibodies. This method can deliver higher-quality antibodies in less time and at lower cost.
Those attributes matter more and more in a time of increasing uncertainty about reproducibility in biology, and particularly with antibodies. From basic research to drug discovery pipelines, scientists are looking for ways to eliminate unwanted variability. Antibody production techniques that eliminate low-quality candidates at the beginning are most likely to provide scientists with reliable, reproducible reagents.
There is also a growing need to accelerate the pace of research. Whether it’s rapidly developing a new immunoassay or speeding up the drug development process, producing higher-quality antibodies faster can help scientists reduce the time for each experiment, or accomplish more iterations within the same time.
Kunhua Chen is CEO of exonbio, a company focused on innovative methods for antibody development.




