Testing nearly 69,000 materials for specific properties was the challenge faced by scientists conducting extensive research on using photocatalytic conversion to reduce the greenhouse gas CO2. The main goal is to find a way to reduce CO2 into chemicals that can provide a source of clean, low-cost renewable energy. But researchers have located very few materials that meet the criteria and the search for new materials is resource intensive, time-consuming and expensive.
A multi-institution research team led by Dr. Kristin Persson as the Primary Investigator worked together to identify new materials that can enable economically viable, industrial-scale CO2 reduction which can be developed into usable fuels. This work was sponsored by the Joint Center for Artificial Photosynthesis which is part of the Department of Energy Innovation Hub which includes researchers from California Institute of Technology, University of California at Berkeley, and the Lawrence Berkeley National Laboratory 1].
Dr. Arunima K. Singh, part of the original research team, is now Assistant Professor of Physics at Arizona State University. “The multi-institution team performed the largest exploratory search to date, covering 68,860 materials, for CO2 reduction photocathodesa with targeted intrinsic properties and identified 43 new photocathode materials which have corrosion resistance, visible-light absorption and an electronic structure which is compatible with fuel synthesis,” Singh explained. “The team used supercomputers to simulate this research and was able to complete the computer simulation in several months. Alternatively, trying to do the simulations on a 250 core cluster computer system would require running the simulation 24 hours a day for at least a year. This work was not possible without using supercomputers.”
Strategy for discovery of new photocathodes.
For an economical, industrial-scale, solar-driven reduction of CO2 a need for new photocatalyst discovery has been noted by researchers. However, most of the search for new photocatalysts is on a trial and error basis. The team looked for suitable photocatalyst surfaces that can supply photo-excited electrons to facilitate the reaction of CO2 with protons in solution. Electrons are excited by the absorption of visible light with a photon energy greater than the bandgap of the photocatalyst material. Electrons of different energies have different thermodynamic propensity for reducing CO2 to different fuels, as shown in Fig. 1.
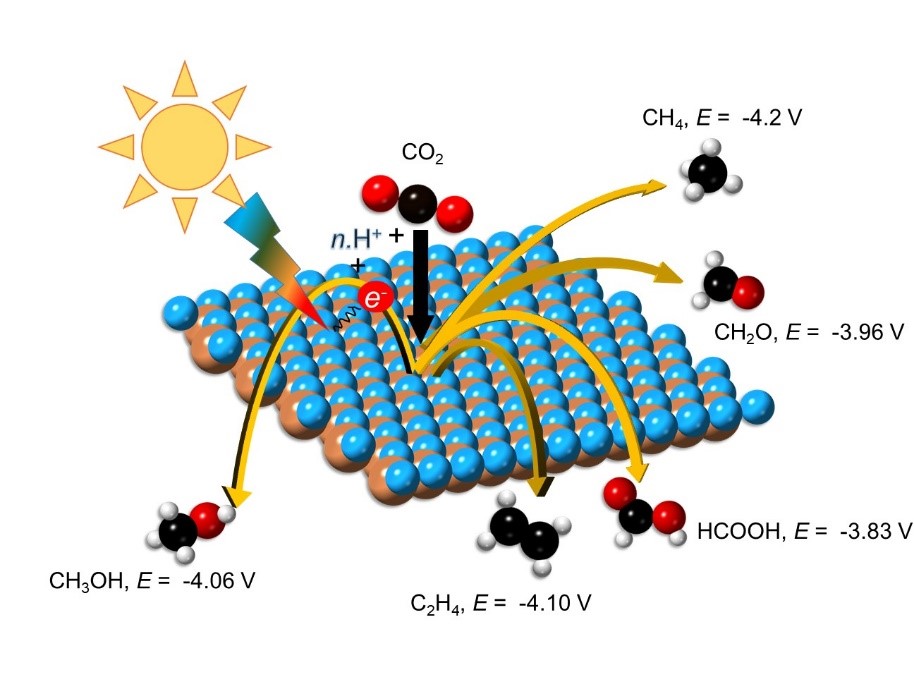
Figure 1. Courtesy of Dr. Arunima K. Singh, Arizona State University.
Search for new photocathodes
One objective of the research was to find new photocathodes that can enable the reaction of CO2 with protons in a water-based (aqueous) solution. Singh indicates, “One of the main challenges in identifying suitable photocathodes is finding materials which exhibit long-term aqueous stability under reducing conditions since most materials reduce to their metallic forms or hydrolyze in water.”
Materials databases aids in research
The team used the open source Materials Project (MP) database that “provides open web-based access to computed information on known and predicted materials as well as powerful analysis tools to inspire and design novel materials.” Research using the MP database has already been used to find sources of new materials for applications such as metallic glasses, electrolytes for batteries and transparent conductors.
The team ran the analysis against 68,860 materials in the MP database. Researchers designed a computational screening strategy to tackle the massive task of computing accurate electronic structure properties used in the research. They prescreened materials based on computed properties available through first-principles simulations-based databases. The research studied the results of materials in six different tiers as shown in Figure 2. Of the six tiers studied, tiers one through four had already been calculated on MP:
- Tier one: Analysis of the first tier estimates the thermodynamic stability of the material and an estimate of the ability to synthesize the material.
- Tier two: The second tier is designed to select materials which have the potential to utilize visible-light.
- Tier three: The 11,507 materials that meet the criteria of tier two were evaluated in this step for stability in a water solution under reducing conditions.
- Tier four: Materials with small lattice structures were selected.
- Tiers five and six: In tier five, the team filtered materials using the hybrid-exchange functional HSE06 to identify materials with bandgap in the visible-light range. In tier six, they evaluated the band edges that show the energies of photo-excited electrons within the solid matter. 2]
The initial research identified 43 materials that merited further investigation for reducing CO2 into fuels. After comparison to literature, eight materials were identified as hypothetical materials. Four of the eight materials did not pass the dynamical stability test, so 39 materials were identified for further research. The results of the simulations were added to the MP database, so the results of this work are available to other researchers.
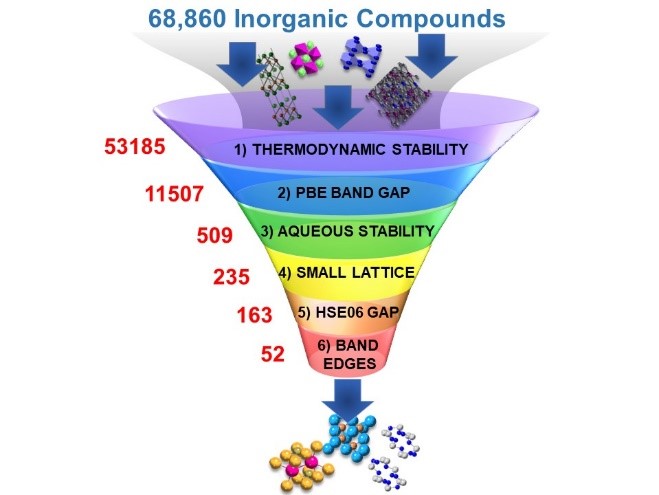
Figure 2. Courtesy of Arunima K. Singh, Arizona State University.
Supercomputers and software used in the CO2 research
The team performed their electronic structure simulations of the large data set containing massive amounts of data partly on the Texas Advanced Computing Center’s (TACC) Stampede2 powered by Intel Xeon Scalable processors with interconnection provided by high-performance Intel Omni-Path Architecture fabric. A significantly larger portion of the research was also executed on the Department of Energy’s National Energy Research Scientific Computing Center (NERSC) Edison supercomputer which is based on Intel Xeon processors and the Cray Aries high-speed interconnect with Dragonfly topology. These systems are some of the most powerful and significant current supercomputers in the U.S. for open science research.
The researchers developed their own formulism to screen for electrochemical stable materials from the 11,507 materials that passed the tier two test. The team used computationally expensive density functional theory (DFT) simulations derived from quantum mechanics as well as hybrid functionals to calculate the overall electronic structure and properties of the materials in tiers 5 and 6. The Vienna Ab initio Simulation Package (VASP) software was used for the quantum mechanical calculations. These computer simulations require highly parallelized code to run efficiently. Intel compilers and the Intel MPI tool were used to help optimize the code and equations.
Results of the initial research
The team’s screening strategy was applied to previous photocathodes research as well as for identifying new photocathode candidates for use as possible clean energy fuels. “We found that our strategy selected materials that are extremely robust in the reducing conditions needed for CO2 reduction. The predicted materials include diverse chemistries such as arsenides, tellurides, selenides, oxides and include several layered materials,” states Singh.
Challenges for Future Research
The Joint Center for Artificial Photosynthesis and computational team continues to perform experimental and computer simulation studies on the 39 photocathodes identified in the original research. On the computational side, the team is evaluating single layer structures, also called two-dimensional materials, to determine whether they have high efficiencies making them suitable for CO2 reduction on an industrial scale.
Singh indicates, “It is only in the last decade that we have been able to calculate the properties of hundreds of thousands of materials. With the supercomputers available today, we can do simulations that look at perfect crystals. However, we cannot currently simulate conditions with impurities or defects in a material—but materials in the real world are seldom without defects and impurities. We need increases in supercomputer capabilities so that we can probe real word conditions to develop solutions in areas such as CO2 reduction to create clean, low cost fuels.”
References
1] Multi-institution team for the research: Joint Center for Artificial Photosynthesis, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA. 2 Chemical Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA. 3 Environmental Energy Technologies Division, Lawrence Berkeley National Laboratory, Berkeley, CA94720, USA. 4 Joint Center for Artificial Photosynthesis, California Institute of Technology, Pasadena, CA 91125, USA. 5 Department of Materials Science and Engineering, University of California, Berkeley, CA 94720, USA. The Primary Investigator for the project was
Kristin A Persson, Lawrence Berkeley Laboratory (LBL). Arunima K. Singh who was interviewed for the project was also at LBL during the research.
2] Robust and synthesizable photocatalysts for CO2 reduction: a data-driven materials discovery, Arunima K. Singh, Joseph H. Montoya, John M. Gregoire and Kristin A. Persson. Published online in Nature Communications, https://www.nature.com/articles/s41467-019-08356-1.pdf
a A photocathode is a negatively charged electrode in a light detection device such as a photomultiplier or phototube that is coated with a photosensitive compound. When this is struck by a quantum of light (photon), the absorbed energy causes electron emission due to the photoelectric effect. – Wikipedia
Linda Barney is the founder and owner of Barney and Associates, a technical/marketing writing, training and web design firm in Beaverton, OR.
###
This article was produced as part of Intel’s HPC editorial program, with the goal of highlighting cutting-edge science, research and innovation driven by the HPC community through advanced technology. The publisher of the content has final editing rights and determines what articles are published.