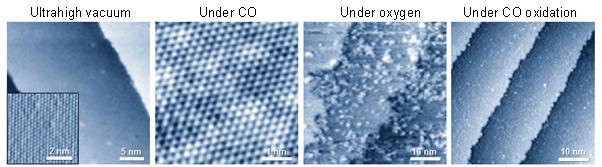
Evolution of surface structures of PtNi bimetal surfaces under various ambient conditions. Credit: KAIST
Catalysts are the result of many chemists searching to unravel the beauty of molecules and the mystery of chemical reactions. Professor Jeong Young Park, whose research focuses on catalytic chemical reactions, is no exception. His research team recently made breakthroughs in addressing long-standing questions for understanding reaction mechanisms on bimetal catalysts.
During the studies reported in Science Advances, following a publication in Nature Communications this month, Professor Park’s research team identified that the formation of metal-oxide interfaces is the key factor responsible for the synergistic catalytic effect in bimetal catalysts. The team confirmed this fundamental reaction mechanism through in situ imaging of reaction conditions. This is the first visualization of bimetal surfaces under reaction conditions, signifying the role of metal-oxide interfaces in heterogeneous catalysis.
Bimetallic materials have outstanding catalytic performance, which opens a new pathway for controlling electronic structures and binding energy in catalysts. Despite considerable research on various catalytic reaction efficiencies, there are yet unanswered questions on the underlying principles behind the improved performance. Even more, it was very hard to figure out what led to the efficiency because the structure, chemical composition, and oxidation state of bimetallic materials change according to reaction conditions.
Recently, some research groups suggested that oxide-metal interfacial sites formed by the surface segregation of bimetallic nanoparticles might be responsible for the increased catalytic performance. However, they failed to present any definitive evidence illustrating the physical nature or the fundamental role of the oxide-metal interfaces leading to the improved performance.
To specifically address this challenge, the research team carried out in situ observations of structural modulation on platinum-nickel bimetal catalysts under carbon monoxide oxidation conditions with ambient pressure scanning tunneling microscopy and ambient pressure X-ray photoelectron spectroscopy.
The team observed that platinum-nickel bimetal catalysts exhibited a variety of different structures depending on the gas conditions. Under ultrahigh vacuum conditions, the surface exhibited a platinum skin layer on the platinum-nickel alloyed surface, selective nickel segregation followed by the formation of nickel oxide clusters using oxygen gas, and finally the coexistence of nickel oxide clusters on the platinum skin during carbon monoxide oxidation. The research team found that the formation of interfacial platinum-nickel oxide nanostructures is responsible for a highly efficient step in the carbon monoxide oxidation reaction.
These findings illustrate that the enhancement of the catalytic activity on the bimetallic catalyst surface originates from the thermodynamically efficient reaction pathways at the metal-metal oxide interface, which demonstrates a straightforward process for the strong metal-support interaction effect. The formation of these interfacial metal-metal oxide nanostructures increases catalytic activity while providing a thermodynamically efficient reaction pathway by lowering the heat of the reactions on the surface. [J. Kim et al. Adsorbate-driven reactive interfacial Pt-NiO1-x nanostructure formation on the Pt3Ni(111) alloy surface, Science Advances (DOI: 10.1126/sciadv.aat3151 ]
Professor Park said that one way to monitor catalysts is to detect hot electrons associated with energy dissipation and conversion processes during surface reactions. His team led the real-time detection of hot electrons generated on bimetallic PtCo nanoparticles during exothermic hydrogen oxidation. The team successfully clarified the origin of the synergistic catalytic activity of PtCo nanoparticles with corresponding chemicurrent values.
By estimating the chemicurrent yield, the research team conclude that the catalytic properties of the bimetallic nanoparticles are strongly governed by the oxide-metal interface, which facilitates hot electron transfer. [H. Lee et al. Boosting hot electron flux and catalytic activity at metal-oxide interfaces of PtCo bimetallic nanoparticles, Nature Comm, 9, 2235 (2018)].
Professor Park explained, “We feel that the precise measurement of hot electrons on catalysts gives insight into the mechanism for heterogeneous catalysis, which can help with the smart design of highly reactive materials. The control of catalytic activity via electronic engineering of catalysts is a promising prospect that may open the door to the new field of combining catalysis with electronics, called “catalytronics.” He added that the study also establishes a strategy for improving catalytic activity for catalytic reactions in industrial chemical reactors.



