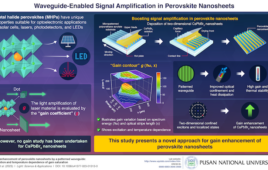
MIT researchers designed nanoparticles that can quickly locate a tumor, then set off a chemical reaction that attracts larger swarms of drug-delivering nanoparticles to the site. Image: Gary Carlson |
For
decades, researchers have been working to develop nanoparticles that deliver
cancer drugs directly to tumors, minimizing the toxic side effects of
chemotherapy. However, only about 1% of the drug typically reaches its intended
target.
Now,
a team of researchers from MIT, the Sanford-Burnham Medical Research Institute,
and the Univ. of California
at San Diego
have designed a new type of delivery system in which a first wave of
nanoparticles homes in on the tumor, then calls in a much larger second wave
that dispenses the cancer drug. This communication between nanoparticles,
enabled by the body’s own biochemistry, boosted drug delivery to tumors by more
than 40-fold in a mouse study.
This
new strategy could enhance the effectiveness of many drugs for cancer and other
diseases, says Geoffrey von Maltzahn, a former MIT doctoral student now at
Cambridge-based Flagship VentureLabs, and lead author of a paper describing the
system in an online edition of Nature Materials.
“What
we’ve demonstrated is that nanoparticles can be engineered to do things like
communicate with each other in the body, and that these capabilities can
improve the efficiency with which they find and treat diseases like cancer,”
von Maltzahn says.
Senior
author of the paper is Sangeeta Bhatia, the John and Dorothy Wilson Professor
of Health Sciences and Technology and Electrical Engineering and Computer
Science and a member of MIT’s David H. Koch Institute for Integrative Cancer
Research.
Harnessing biology
Von Maltzahn and Bhatia drew their inspiration from complex biological systems
in which many components work together to achieve a common goal. For example,
the immune system works through highly orchestrated cooperation between many
different types of cells.
“There
are beautiful examples throughout biology where at a system scale, complex
behaviors emerge as a result of interaction, cooperation, and communication
between simple individual components,” von Maltzahn says.
The
MIT team’s approach is based on the blood coagulation cascade—a series of
reactions that starts when the body detects injury to a blood vessel. Proteins
in the blood known as clotting factors interact in a complex chain of steps to
form strands of fibrin, which help seal the injury site and prevent blood loss.
To
harness the communication power of that cascade, the researchers needed two
types of nanoparticles: signaling and receiving.
Signaling
particles, which make up the first wave, exit the bloodstream and arrive at the
tumor site via tiny holes in the leaky blood vessels that typically surround tumors.
Once at the tumor, this first wave of particles provokes the body into
believing that an injury has occurred at a tumor site, either by emitting heat
or by binding to a protein that sets off the coagulation cascade.
Receiving
particles are coated with proteins that bind to fibrin, which attracts them to
the site of blood clotting. Those second-wave particles also carry a drug
payload, which they release once they reach the tumor.
In
a study of mice, one system of communicating nanoparticles delivered 40 times
more doxorubicin than non-communicating nanoparticles. The researchers also saw
a correspondingly amplified therapeutic effect on the tumors of mice treated
with communicating nanoparticles.
To
pave the path for potential clinical trials and regulatory approval, the MIT
researchers are now exploring ways to replace components of these cooperative
nanosystems with drugs already being tested in patients. For example, drugs
that induce coagulation at tumor sites could replace the signaling particles
tested in this study.