By Nancy Westcott, President of GoatThroat Pumps In laboratory environments, hazardous chemicals such as solvents, acetone, acids and cleansers are frequently transferred from large drums or containers into smaller vessels such as Erlenmeyer flasks or beakers. Given that many of these chemicals and branded formulations are flammable, hazardous or potentially combustible, environmental, health and safety…
Safe coronavirus research with Systec autoclaves
The coronavirus COVID-19, which first appeared in the Chinese province of Wuhan in December of 2019, spread to all continents of the world within just a few weeks. National and international authorities are currently managing efforts to contain the virus by deliberately interrupting infection chains. At the same time, the virus is being examined at…
Lightning protection for laboratories prevents lost research and equipment
By Jennifer Morgan and Michael Chusid “What about the mice?” That was the first response of a postdoctoral fellow when asked what a lightning strike could do to her lab at a major biomedical research institute. In addition to electrocution, fire, and other animal welfare concerns, she is worried about the thousands of hours…
Study finds doctor-pharmacist partnership significantly reduces patient harm
Medication errors are among the most common incidents reported in hospitals, often occurring at hospital admission, with a new study led by Monash University and Alfred Health researchers evaluating a collaborative model to reduce medication errors and length of hospital stay. The study of 8648 patients found that having a partnered pharmacist medication charting* (PPMC)…
Massive Recall Issued for 12 Million Pounds of Beef
The U.S. Department of Agriculture has announced that 12 million pounds of non-intact raw beef products are being recalled, due to concerns about possible salmonella contamination. JBS Tolleson Inc. of Tolleson, Ariz. is issuing the recall for the beef items, including ground beef, which were packaged dates spanning from July 26, 2018 to Sept. 7,…
New International Standard for Fungi, Bacteria Testing
A new ASTM International standard will help minimize microbial contamination of culture plate media and sampling devices used in indoor air quality investigations. The standard describes how to sample and transport culturable airborne fungi and bacteria samples using agar plates. “This standard is valuable when species-level identification or quantity of fungi and bacteria are important…
USP’s Overhaul of Compounding Rules Continues
Earlier this year, the United States Pharmacopeia (USP) took the first in a series of steps to revise the standards for compounding nonsterile and sterile preparations, and aligning the relevant chapters with USP <800> (“Hazardous Drugs — Handling in Healthcare Settings”), which was published in 2016 and goes into effect December 1, 2019. The public comment period for USP <795> closed in…
Understanding the Compliance Expectations for the Falsified Medicines Directive
Manufacturers and distributors of counterfeit medicines are becoming more sophisticated in terms of their ability to enter the legitimate pharmaceutical supply chain. In 2011 the European Council amended Directive 2001/83/EC to define the rules for manufacturing, importing, placing on the market, the wholesale distribution of medicinal products in the Union, as well as rules relating to active substances, by issuing the European…
Standards Group Issues Graphene Use Guidance
Over the last decade there has been a huge interest in graphene, commercially and scientifically, due to the many exceptional properties associated with the material. Graphene is the world’s strongest and thinnest material, and an excellent conductor of heat and electricity. BSI, the business standards company, has published a new guide to the properties of…
EPA May Change Calculation Method for Financial Effects of Regulations
The way that the Environmental Protection Agency determines the costs and benefits of regulations is under scrutiny and could soon change, according to an article in Chemical & Engineering News (C&EN), the weekly newsmagazine of the American Chemical Society. Since the 1980s, federal agencies — including EPA — have calculated the financial costs and benefits…
FDA Investigating Possible Salmonella Contamination of Popular Ingredient
The U.S. Food and Drug Administration, the Centers for Disease Control and Prevention, the U.S. Department of Agriculture, along with other partners, are investigating possible Salmonella contamination of a whey ingredient that has caused the recall of several popular foods. Associated Milk Producers Inc. (AMPI) of New Ulm, Minn., is recalling dry whey powder due…
USP’s Update to Compounding Rules Could Affect Cleanroom Design
For facilities with cleanrooms, the guidance from the United States Pharmacopeia (USP) is likely already quite familiar. Rules for cleanroom design are covered at length in USP General Chapter <800> (“Hazardous Drugs — Handling in Healthcare Settings”), as well as General Chapters <795> (“Pharmaceutical Compounding — Nonsterile Preparations”) and <797> (“Pharmaceutical Compounding — Sterile Preparations”).…
Data Integrity: Compliance with GMP and FDA Requirements
Data integrity is an old concept made essential in today’s digital age, and means data that is accurate, complete, and repeatable, which in turn ensures the product’s quality and public safety. In recent years, infractions relating to data integrity have been noted in several Food and Drug Administration (FDA) warning letters. The importance of record-keeping in drug manufacturing can be seen as…
FDA’s Draft Guidance of “Least Burdensome Provisions” for Medical Devices
In 1997, Congress passed the Food and Drug Administration Modernization Act of 1997 (FDAMA)1 in an effort “to ensure the timely availability of safe and effective new products that will benefit the public and to ensure that our Nation continues to lead the world in new product innovation and development.” Central to that legislation was the addition of section 513(i)(1)(D) and 513(a)(3) (D)(ii)…
New Nanotech Workplace Recommendations Released
Realizing the promise of any scientific advancement requires understanding of its potential human health effects, and its safe and responsible development, even at the level of engineered nanomaterials, which can be nearly atomic-sized. The National Institute for Occupational Safety and Health (NIOSH) has launched four new products this week intended to provide options to companies…
FDA Updates Guidance on Electronic Records, Signatures
After issuing its controversial Guidance for Industry — Part 11, Electronic Records; Electronic Signatures — Scope and Application1 20 years ago, the FDA struggled with consistent enforcement of the guidance. At the time, industry countered with feedback that the new guidance presented an overhead model that was untenable from a business perspective. We have gained little insight into the FDA’s thinking since their…
Disposable Diapers Enhance Tumor Growth Measurements
Catching cancer early can make all the difference for successful treatment. A common screening practice measures tumor growth with X-ray computed tomography (CT), which takes a series of cross-section images of the body. Before they are used in clinics, researchers test multiple CT imaging techniques with standard objects called “phantoms,” designed to mimic real tumors.…
First Graphene ISO Standard Published, Will Heighten Commercialization
The world’s first ISO (International Organization for Standardization) graphene standard has been published. The standard will provide consistency across the emerging world-wide graphene industry and accelerate the 2D material’s commercial exploitation. The new international standard, led by the National Physical Laboratory (NPL), defines the terminology used to describe the many different forms of graphene and…
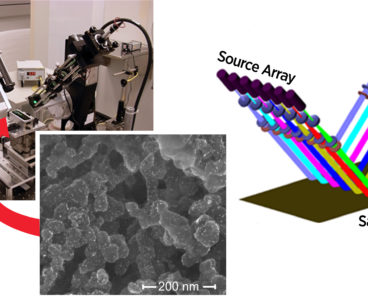
Fuel Cell “Printing” Measured Quickly with New Technique
If you’re wondering when a hydrogen-powered car will become a viable option for you, take heart. A team including scientists at the National Institute of Standards and Technology (NIST) may have overcome a significant hurdle to manufacturing hydrogen fuel cells by creating a way to check whether the expensive catalysts the cells need have been…
Preparing for the New European Medical Device Regulations
The European parliament published new regulations for medical devices (MDR) and in-vitro diagnostics (IVDR) on May 5, 2017, capping almost eight years of negotiations between the European Union Council, Parliament, and Commission. Each regulation was published in the European Official Journal on May 25, 2017. The motivation to update the existing directives stemmed from inconsistent…
Statistical Process Control in Cleanrooms for Chip, Solar Cell Manufacturing
Today, Statistical Process Control (SPC) is the gold standard of quality control because it helps manufacturers to maximize production of on-spec product with minimal waste and rework. SPC uses graphical control charts to determine when a process should be adjusted. The following guidelines can help silicon chip and solar cell manufacturers make the best use…
Selecting the Right Monitoring Instruments for Cleanroom Classification
One of the biggest challenges in any cleanroom is setting up a suitable monitoring program. Which instruments are used, how frequent samples should be, how much sampling is required, what guidelines need to be followed? All of these questions need a firm understanding of the different technologies capturing cleanroom air samples in portable particle counters…
The Value of Traceability
In the pharmaceutical industry, the traceability of prescription drugs is increasingly vital to the integrity of pharmaceutical products. By providing more end-to-end visibility into pharma supply chains, companies can better trace serialized products from wholesalers to end customers, enhancing the reliability of supply deliveries while also offering a way to detect counterfeit drugs and track…
New Survey Reveals the Importance of Standardized Label Management in Pharmaceutical Industry
One of the overarching themes emerging from the survey was the need for a single, centralized platform for label design, change control, printing, integration and management to overcome the challenges: “Only 14% of those surveyed have achieved 100% integration with their MES/ERP system. Over 34% are still using separate systems to manage their labeling and…
Defining Durability in Industrial Labels: Why the Right Label Matters
Although standard “office” labels are more than sufficient for routine applications like filing, addressing envelopes, and shipping boxes, they are not designed to withstand the range of conditions and hazards found in harsh industrial settings such as warehouses, production lines, laboratories, or construction sites. Fortunately, film labels are available that are engineered to withstand harsh…