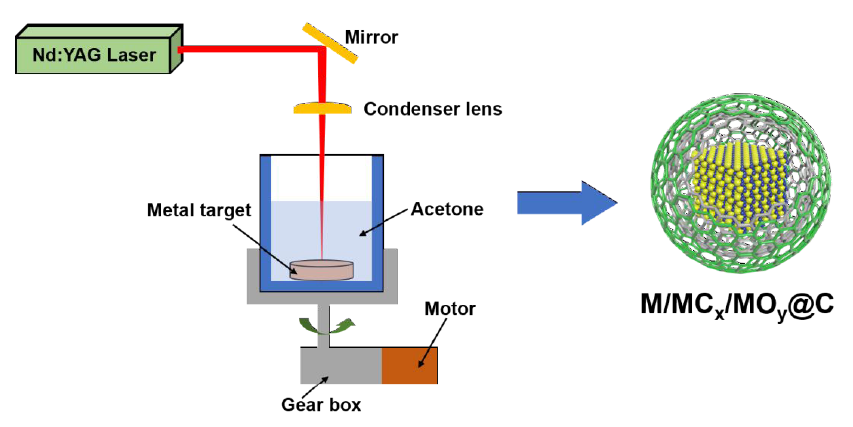
illustration of the LAL Technique and Schematic of the Final Products. Image: Zhang Chao
Chinese researchers revealed the reason for forming M/MCx/MOy@C in LAL technology through studies led by LIANG Changhao of Institute of Solid Physics, Hefei Institutes of Physical Science.
Recently, carbon encapsulated nanomaterials have triggered tremendous efforts due to their outstanding performance in thermocatalytic or electrochemical catalytic reactions.
Laser ablation of metal in organic solvents (LAMOS) has been proven to be an efficient technique for one-step synthesis of carbon-encapsulated metal/metal carbide/metal oxide core-shell nanostructures.
Why are the core compositions out of step for different metals in the same technology? How do amorphous and graphite carbon shell evolve during the progress of LAMOS?
To find out the reasons behind, the scientists selected acetone as the representative solvent and 16 transition-metal targets at the same time, including Cu, Ag, Au, Pd, Pt, Ti, V, Nb, Cr, Mo, W, Ni, Zr, Mn, Fe and Zn.
By LAMOS, the final products could be divided into three types, including carbon encapsulated metals (M@C), carbon encapsulated metal carbides (MCx@C) and carbon encapsulated metal/metal oxides (M/MOy@C).
They found that the carbon solubility in metals and the affinity of metals to oxygen were the critical factors in determining the core composition, while metal catalyzed carbonization determined the state of the carbon shells with different crystallization rates.
In addition, they performed a designed experiment toward Pt, through which they indicated that the metal catalyzed carbonization played a crucial role in the state of the carbon shells.
This research was supported by the National Basic Research Program of China, the Instrument Developing Project of the Chinese Academy of Sciences, the National Natural Science Foundation of China and CAS/SAFEA International Partnership Program for Creative Research Teams.




