Imaging very small materials takes not only great skill on the part of the microscopist, but also great instruments and techniques. For a refined microscopic look at biological materials, the challenges include getting an image that is free from “noise,” the interference that can be caused by a number of items, including the area surrounding an item. Labels, dyes, or stains that are added in order to see the item more clearly can also present issues as they can affect the item that is to be scanned in unexpected ways–damaging or even killing biological materials.
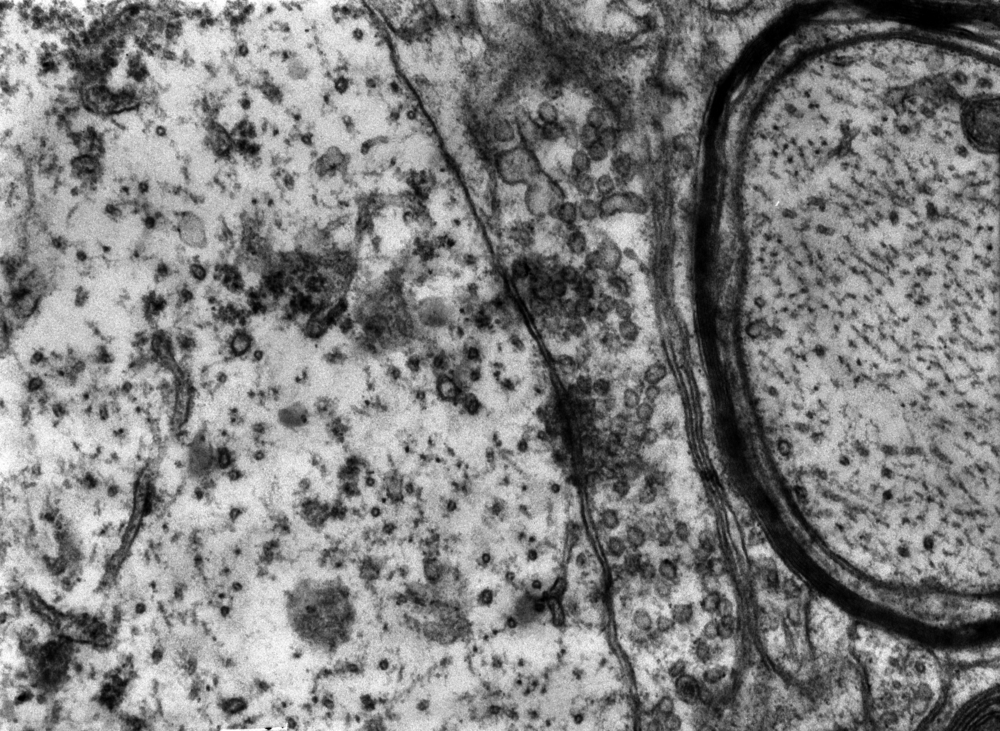
Looking at microtubules is an interesting case in point. The hollow tubular structure serve as a backbone of cells and helps carry materials in the cell. Malfunctioning microtubules have been associated with various illnesses including cancer and Alzheimer’s disease.
Understanding how microtubules function could be an important step in understanding disease progression. However, studying a single dynamic microtubule, which measures 24 nanometers in diameter, and up to 10 microns in length, is not an easy task.
Researchers in the Quantitative Light Imaging Laboratory at the Beckman Institute for Advanced Science and Technology at the University of Illinois have been able to use label-free spatial light interference microscopy (SLIM) and computer processing in order to image the microtubules in an assay. The study, “Label-Free Imaging of Single Microtubule Dynamics Using Spatial Light Interference Microscopy,” was recently published in ACS Nano.
Being able to see the microtubules without the use of dyes or stains is a major contribution.
“The label-free aspect is the main breakthrough in my opinion,” said Gabriel Popescu, associate professor of electrical and computer engineering, and member of Beckman’s Bioimaging Science and Technology Group. Popescu is the senior author on the study.
“There have been other efforts towards making this label-free, it’s a very important class of challenges. Current techniques yield smaller fields of view, and the image contrast is not as good.”
By measuring how much light is delayed through the specimen at all points in the field of view, the researchers are able to find the optical path length map for the sample. This optical path length–or phase information–relates to a sample’s refractive index and thickness, enabling detailed studies on cell structure and dynamics.
“The instrument provides a blurring of the image that’s much bigger than the size of the microtubule,” explains Popescu. “So it’s as if it’s smearing out the values of that phase delay. But since we our system very well, we’re able to back it up and come up with an effective index value for the microtubule, which is correct.”
The numerical processing used provides the sensitivity not only to see the tubules but also is used to measure light scattering.
“A key physics point is that once you know both the intensity and phase of the light, then you can numerically process that information and virtually propagate the light anywhere in space, including at a plane far away from the microtubule, in order to study the scattered light,” said Popescu.
Previous efforts at imaging the miniscule structures have used immunofluorescence, injecting antibodies into fluorescent dyes in order to clearly see the cell as it functions. However, the fluorescence can affect cell function and the length of time that the cell can be imaged.
“We imaged them for a very long period of time, not two or three minutes, but more like eight hours,” said Mikhail Kandel, a doctoral student in electrical and computer engineering and lead author on the study. “People are interested in the metabolic rates of the proteins that walk on the microtubules and we showed how you can watch the deceleration of these proteins, which is equivalent to monitoring the consumption of their fuel source.”
“You could potentially figure out the consumption of ATP and motility characteristics of the proteins, which are very interesting.”
The Beckman researchers worked with Paul Selvin, professor of physics.
“This just came out of a discussion with Paul Selvin’s group, who have been studying microtubules for a long time using traditional methods of fluorescence,” said Popescu. “Mikhail got in contact with his students and they said, let’s give it a try. Seeing them with other types of fluorescence is a major improvement because you can basically image them forever.”
“My group is interested in seeing how proteins move on and around microtubules,” said Selvin, one of the study’s authors. “This new technique not only enables us to get an idea of how the cells will function over time, but also raises the possibility of in vivo imaging of cells.”
SLIM is a commercially manufactured product that can fit on to upgrade about any microscope, say the researchers. This allows biologists to use other microscopy techniques, including fluorescence, in addition to SLIM. The SLIM product is available through Phi Optics, a company that Popescu founded.
“One of the biggest challenges in interferometry is sensitivity, which is affected drastically by environmental noise, for example, vibrations or air fluctuations. But with the particular stable geometry used in SLIM, we can actually achieve incredible sensitivity in fractions of nanometers,” said Popescu.
The researchers plan to push the boundaries on imaging cells, hopefully imaging microtubules in live cells.
“If we manage to push this in a living cell, that would be a real breakthrough,” said Popescu. “We anticipate great challenges because of the background that exists in the cells. Encouraged by these results, we are thinking that one day we might be able to have such a sensitivity to see phase shifts from single molecules.
“We’re not there yet, but one can dream.”