Cerenovus President Mark Dickinson forecasts the innovative technologies that will advance stroke care in the coming years.

Cerenovus Worldwide President Mark Dickinson [Photo courtesy of Johnson & Johnson MedTech]
It’s getting harder to beat aspiration systems for fast and simple thrombectomies to remove blood clots that are blocking oxygen from a stroke patient’s brain.
That’s according to Cerenovus Worldwide President Mark Dickinson, who discussed the future of stroke care in an interview with Medical Design & Outsourcing.
“It’s a very simple concept that the larger the tube you can get to face the blood clot, the more likely you’re going to be able to evacuate that clot just through suction,” he said. “And advances in technology have enabled us — and, candidly, others in the marketplace — to be able to design these larger bore, larger sized devices to get to destinations that they would never have been able to get to before.”
While there will still be stubborn clots that call for stent retrievers, aspiration is a simpler technique that requires less skill, which could help expand access to stroke treatment in the U.S. and abroad. Great engineering is only partially responsible for getting thrombectomy treatment as far as it’s advanced, he said.
“It’s mostly through highly skilled physicians being able to treat these patients,” he said. “But there are just so many people out there who are having a stroke. … By 2050, almost 10 million people a year are going to die from a stroke, so we really need technologies and methodologies that the masses can use and not just be reliant upon the skills of the 100 top doctors around the world.”

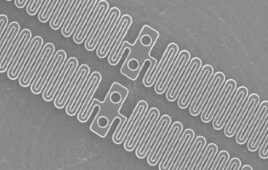
Cerenovus designed the Cereglide71 catheter for direct aspiration and up to three stent retriever passes. [Image courtesy of Johnson & Johnson MedTech]
Cerenovus — a unit of Johnson & Johnson MedTech, the world’s second-largest device manufacturer — recently launched the Cereglide 71 catheter for direct aspiration as well as delivery of the Cerenovus Embotrap clot retriever. This next-generation aspiration catheter is the first aspiration device that Cerenovus has developed through the efforts of its Neuro Thromboembolic Initiative (NTI) scientific research arm.
“Anybody can make a device and make it track well in one of these simple anatomical models that we traditionally would test these devices on, and then you take that device to the real world and it doesn’t perform to the level that either we would hope for or our customers are expecting,” Dickinson said. “[We] put these products through the most physical tests we possibly can so when we take them to a patient, we’ve got high confidence that we’re going to be successful. … The anatomy is so complicated. It’s so varied. And it can be challenging from one patient to the next.”
Like other neurovascular device developers, Dickinson envisions robotics and remote technology expanding access.
“At J&J we’ve got massive investment going into our robotic technologies,” he said. “At the moment, it’s focused more on general surgery and orthopedics. But there’s definitely potential for crossover into the future. We’ll see that evolve. It’s going to take time for that to be a really meaningful player in terms of procedure volumes in the neurovascular space, but for sure it will come.”
Until that time, Dickinson said, “our objective in the short- to medium-term is just to make sure that the device that gets to the clot can effectively remove the clot. The robotics piece — we can plug into those things in the future as needed.”
Dickinson offered a taste of other potential advances in stroke care. The Cerenovus team in Galway, Ireland, has a couple of research projects looking into whether sensors on the tip of a catheter could use light spectroscopy or impedance measurements to see into a clot and help determine the best way to treat it.
However, physicians would prefer a single device that can handle any clot quickly, he said. “Ultimately, we want to design a device that can be completely agnostic to the type of clot.”
There’s currently an arms race for super-bore catheters that can get into the M1 segment of the middle cerebral artery, which is the most common artery for stroke-causing blood clots.
But the winning solution will likely be more of a system than just a catheter. The big differentiator after catheter design, Dickinson thinks, will be in the rest of the system with features such as cyclic aspiration, also called intermittent or pulsatile aspiration. Instead of binary on/off suction to remove the clot, cyclic aspiration rolls the vacuum through different cycles to disrupt particularly stubborn clots to make them easier to remove.
“That’s where you’re going to see the most innovative changes in the space in the next five years,” he said. “There’s a lot of research and development going on in that space right now. And I know others are looking at that as well. … We’re seeing a very strong signal in terms of our ability to remove clots that we know from our clot science work with previous techniques have been very difficult to budge, so we’re pretty excited about it.”
He declined to share more details, citing a need to protect proprietary information.
Asked for advice he would share with other medical device developers from his medtech career, he offered an old adage that “still remains very true.”
“Fail fast,” he said. “Bring your concept quickly and find the right way to test it [with] benchtop models and test methodology to really put something through its paces. Do that early. And if you’re failing, scrap and start and move on to the next one.”
“Speed to market is so important, so don’t waste time,” he continued. “If something’s not working, get on to the next thing.”