Researchers at AMOLF have found a way of making calcium carbonate structures, such as a sea urchin skeleton, suitable for use in electronics. They do this by modifying the composition of the material so that it becomes a semiconductor without losing its shape. This could lead to more efficient and stable solar cells. This research was published in the journal Nature Chemistry on June 4.
In principle, you could carry out the experiment on the beach using a cuttlebone (white oval shell of a cuttlefish) or a sea urchin skeleton, says Wim Noorduin, group leader Self Organizing Matter at AMOLF. “The experiment involves no more than dripping two different liquids over the calcium carbonate structure. The conversion is complete within a couple of minutes. If you shine a UV lamp on the structure, you can see the conversion taking place in front of your eyes: the sea urchin skeleton which initially appears blue under the lamp changes into a bright green structure with each drop.”
Noorduin converts calcium carbonate structures such as a sea urchin skeleton into perovskite, a highly promising novel material for solar cells. “In effect, this is alchemy,” says Noorduin. “Midas changed everything into gold, and we are now changing calcium carbonate into perovskite.”
Calcium carbonate is highly abundant on earth and can be found in chalk mines and animal skeletons, for example. Noorduin had previously found a way of making a range of different microstructures from calcium in order to understand how nature does that. But the material has few applications.
Perovskite, however, offers more possibilities, and it is a highly promising novel material for solar cells. It is the “gold” within semiconductor research, says Noorduin. Solar cells produced from the semiconductor perovskite are efficient and cheaper than traditional silicon solar cells. They are also the subject of increasing amounts of research.
“By converting a predetermined structure of calcium carbonate into the functional perovskite, we now have control over both the shape and function of the material,” says Noorduin.
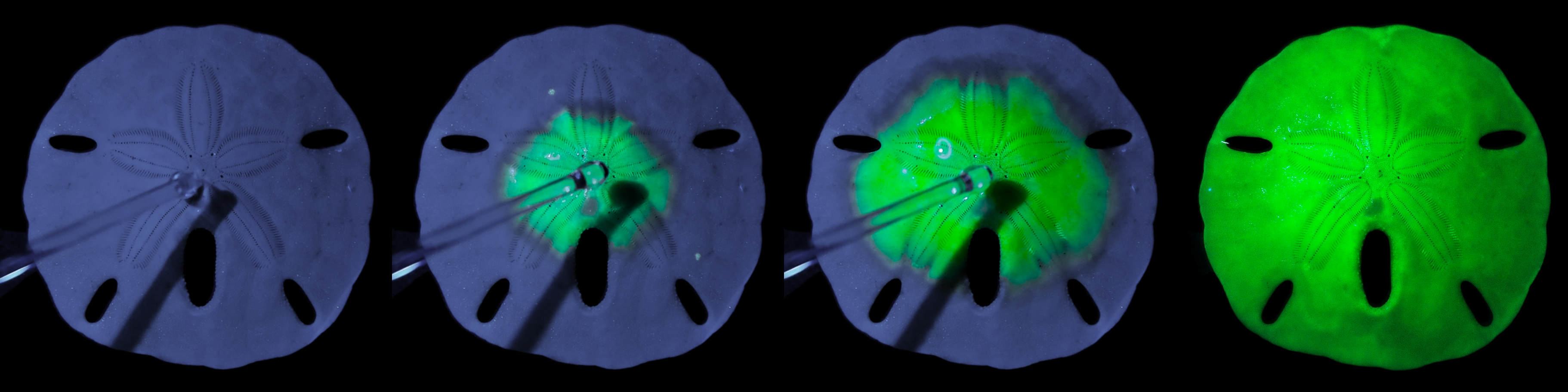
This series of pictures shows a sand dollar skeleton gradually converting into a light emitting perovskite. Image: Noorduin Lab, AMOLF
Noorduin expects the new material will lead to improved solar cells. As the researchers now have control over the shape of the solar cell, they can produce a structure that captures sunlight more effectively. Furthermore, the lifetime of the current generation of solar cells made from perovskite is too short. Perovskite degrades too quickly.
“We think that our perovskite microstructures are far more stable. Solar cells made from this material should therefore last for longer,” says Noorduin. “In addition, we can produce perovskite structures in every desired color. This means that the material could also be used for LEDs in various applications, such as screens.”
With the new process, developed by Noorduin’s PhD researchers Lukas Helmbrecht and Hans Hendrikse, it is possible to convert every calcium carbonate structure, such as a sea urchin skeleton or Noorduin’s microstructures, into perovskite. This process concerns the controlled conversion of one crystal structure into another, which is a difficult process in chemistry. A crystal structure is similar to a collection of stacked marbles, where the marbles represent the ions. The ions in calcium carbonate are different from those in perovskite, and the stacking is different as well. The researchers replace all the ions in the calcium carbonate: first the positively charged calcium ions with lead irons, and then the negatively charged carbonate irons with chloride, for example. Finally, they add another ion, the methyl ammonium ion. This last ingredient gives rise to a new stacking pattern as a result of which perovskite is produced.
The experiment is simple, once you know how to perform it, says Noorduin. The difficulty of converting calcium carbonate into perovskite is that everything is different: not just the composition of positively charged cations and negatively charged anions, but also the crystal structure, says Noorduin.
“The reaction conditions, such as concentration and pH level, must be exactly right, as otherwise the structure falls apart immediately. It took us six months to discover those exact conditions.”
For example, the exchange of the cations in the first step must be perfect. The second step is even more difficult because the crystal structure must change. We also found it was vital to ensure that this last step occurs very quickly to prevent the structure from falling apart.
The ion exchange method can be used on a wide range of materials. Not only calcium carbonate, but also barium carbonate and strontium carbonate are suitable, and possibly sulfates as well.
The AMOLF researchers expect that the reaction can also be expanded to other types of perovskite to make a wide range of applications possible. “We can apply the principles to other materials such as catalysts. In those cases, you want to be able to control the material’s surface shape and composition as well.”
Source: AMOLF