National University of Singapore scientists have developed design guidelines that increase the catalytic effectiveness of graphene-based solid state catalysts for potential industry applications.
Catalysts are widely used in the chemical industry to make manufacturing processes more efficient and economical. This is achieved by providing an alternative pathway for the synthesis of chemicals and compounds. Graphene-based solid state catalysis (GBSSC) is an emerging research direction, which opens new opportunities for graphene applications in the production of chemicals. Graphene has a very high surface-to-volume ratio and is therefore expected to be a promising candidate for catalysts. However, as graphene itself is chemically inert, an effective yet practical way is required to activate and unlock its catalytic potential. Many methods to activate graphene have been proposed in literature. These methods include introducing dopants, creating mechanical strains and appending functional groups to it. As these methods require direct treatment of graphene (by modifying its structure or chemical composition), they are difficult, if not impossible, to realize in a controllable manner due to graphene’s highly inactive nature. This limits the use of GBSSC for large-scale production in industrial applications.
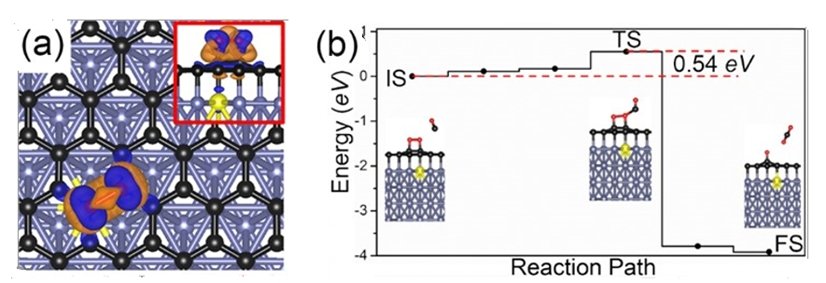
Figure shows (a) charge transfer between the supported graphene and the adsorbed O2 molecule when the substrate is doped with an impurity (in this example, a metal element, colour coded in yellow). Blue (brown) colour denotes the accumulation (depletion) of electrons. (b) Energy profile of the carbon monoxide oxidation process catalysed by graphene supported on a metal-doped substrate (CO + O2⇒CO2 + O*). In the presence of the graphene catalyst, the reaction barrier is lower at 0.54 eV. Without the graphene catalyst, it is much higher at more than 3 eV. The computed atomic structures at various state of the process is shown (IS: initial state, TS: transition state, FS: final state). Image: npj 2-D Materials and Applications
Using computational modelling and simulation techniques, Professor Zhang Chun and his research team from both the Departments of Physics and Chemistry, NUS have developed a way of activating graphene by using defects in the underlying substrate. These defects include doped impurity atoms or vacancies. This method avoids direct treatment of graphene, making it a much more practical way to unlock its catalytic potential for industrial applications. Using intensive and comprehensive ab initio calculations (using basic principles), the research team showed that certain types of defects in the substrate (substitutional metal impurity atoms or vacancies) can greatly enhance the reactivity of supported graphene. This resulted in strong chemical adsorption of oxygen molecules on the graphene and drastically lowered the barriers for catalyzed carbon monoxide (CO) oxidation reactions.
Zhang explains, “The origin of the high reactivity and catalytic activity is found to be driven by the impurity- or vacancy-induced charge transfer from the graphene-substrate contact region to the oxygen 2π orbital. This charge transfer weakens and facilitates the breaking of the oxygen-oxygen (O-O) bond of the oxygen (O2) molecule which is adsorbed on the graphene sheet and enables the formation of carbon dioxide (CO2). Without the charge transfer, the O-O bond is too strong for the CO oxidation reaction to take place under room temperature. Our results pave the way for a new family of high performance graphene-based solid state catalysts with the potential for industrial applications.”
The team plans to collaborate with experimentalists to fabricate the proposed catalysts and explore the possibility of large-scale applications.
Source: National University of Singapore