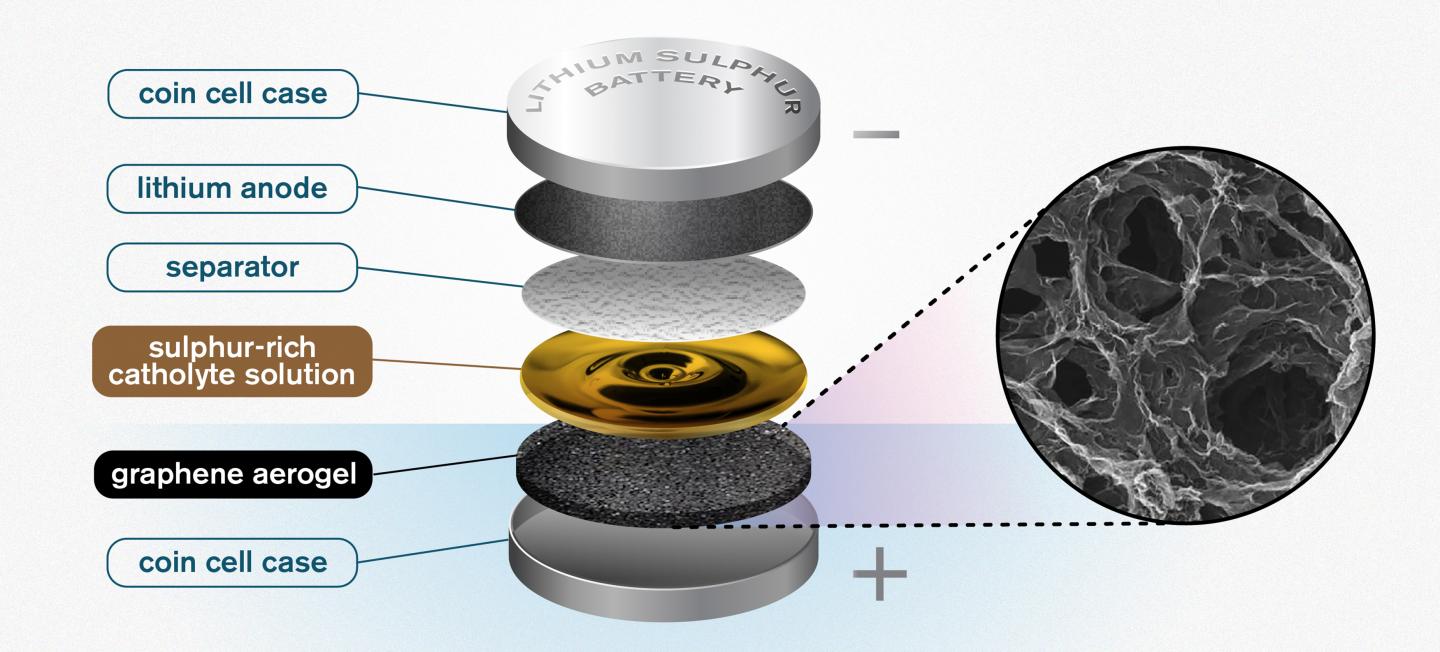
An illustration of the Chalmers design for a lithium sulfur battery. The highly porous quality of the graphene aerogel allows for high enough soaking of sulfur to make the catholyte concept worthwhile. Credit: Yen Strandqvist/Chalmers University of Technology
Scientists from the Chalmers University of Technology in Sweden believe a new type of battery with a catholyte that contains a graphene sponge could help bring lithium-sulphur batteries to the forefront, eventually replacing lithium-ion batteries.
The researchers found that a porous, sponge-like aerogel made from reduced graphene oxide acts as a freestanding electrode in the battery cell, allowing for better and higher utilization of the sulphur with more than five times the energy density of lithium-ion batteries.
Traditional batteries consist of two supporting electrodes—an anode and cathode—coated with an active substance, a liquid electrolyte that allows ions to be transferred back and forth and a separator that acts as a physical barrier that prevents contact between the two electrodes while still allowing ions to transfer.
In previous studies, the researchers looked at combining the cathode and electrolyte into a liquid called a catholyte to reduce battery weight, while increasing the speed of charging and adding power capabilities.
The researchers found that by adding a thin layer of a porous graphene aerogel that acts as a sponge to soak up the sulphur-rich catholyte, they could improve the battery even further.
“You take the aerogel, which is a long thin cylinder, and then you slice it—almost like a salami,” Carmen Cavallo of the Department of Physics at Chalmers and lead researcher on the study, said in a statement. “You take that slice, and compress it, to fit into the battery. The porous structure of the graphene aerogel is key. It soaks up a high amount of the catholyte, giving you high enough sulphur loading to make the catholyte concept worthwhile. This kind of semi-liquid catholyte is really essential here. It allows the sulphur to cycle back and forth without any losses. It is not lost through dissolution – because it is already dissolved into the catholyte solution.”
The researchers also applied some of the solution to the separator to fulfil its electrolyte role and maximize the sulphur content in the battery.
Lithium-ion batteries, which are commonly used in virtually every electrical device and vehicle, are nearing the end of what they are capable of in keeping up with modern devices that are requiring more and more energy to run. Researchers have been in recent years scrambling to find a replacement, zeroing in on lithium-sulphur batteries with a theoretical energy density of 1000-to-1500 watt-hours per kg, significantly more than the current limit of 300 watt-hours per kg in lithium-ion batteries.
“Furthermore, sulphur is cheap, highly abundant, and much more environmentally friendly,” Aleksandar Matic, Professor at Chalmers Department of Physics, who leads the research group behind the paper, said in a statement. “Lithium-sulphur batteries also have the advantage of not needing to contain any environmentally harmful fluorine, as is commonly found in lithium-ion batteries.”
While the benefits are numerous, there have been some negatives associated with lithium-sulphur batteries that have prevented their implementation, including instability and low cycle life. However, the new prototype demonstrated an improved 85 percent capacity retention after 350 cycles by decreasing the sulphur that dissolves into the electrolyte and avoiding the shuttling effect, where Sulphur molecules migrate from the cathode to the anode.
While the new technology shows promise, the researchers will still need to develop new manufacturing processes to make the batteries commercially viable.
The study was published in the Journal of Power Sources.




