Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. These chemical compounds are usually organic molecules or gases. For the GC to be successful in their analysis, these compounds need to be volatile, usually with a molecular weight below 1250 Da, and thermally stable so don’t degrade in the GC system.
The process of GC involves separating compounds in a mixture by injecting a gaseous or liquid sample into a mobile phase, typically called the carrier gas, and passing the gas through a stationary phase. The mobile phase is usually an inert gas or an unreactive gas such as helium, argon, nitrogen or hydrogen. The stationary phase is a microscopic layer of viscous liquid on a surface of solid particles on an inert solid support inside a piece of glass or metal tubing called a column. The surface of the solid particles may also act as the stationary phase in some columns. The glass or metal column through which the gas phase passes is located in an oven where the temperature of the gas can be controlled and the eluent coming off the column is monitored by a computerized detector.
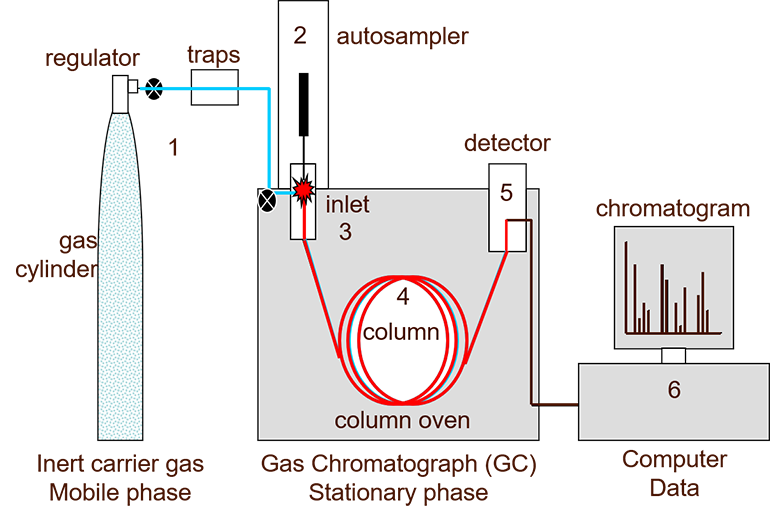
Figure 1: A simplified diagram of a gas chromatograph showing: (1) carrier gas, (2) autosampler, (3) inlet, (4) analytical column, (5) detector and (6) PC. Credit: Anthias Consulting.
GC is widely used in most industries for quality control in manufacturing cars, chemicals, pharmaceuticals; for research: the analysis of meteorites to natural products; and for safety: environmental to food to forensics.
Gas chromatography is also sometimes known as vapor-phase chromatography (VPC), or gas–liquid partition chromatography (GLPC). These alternative names, as well as their respective abbreviations, are frequently used in scientific literature.




Tell Us What You Think!