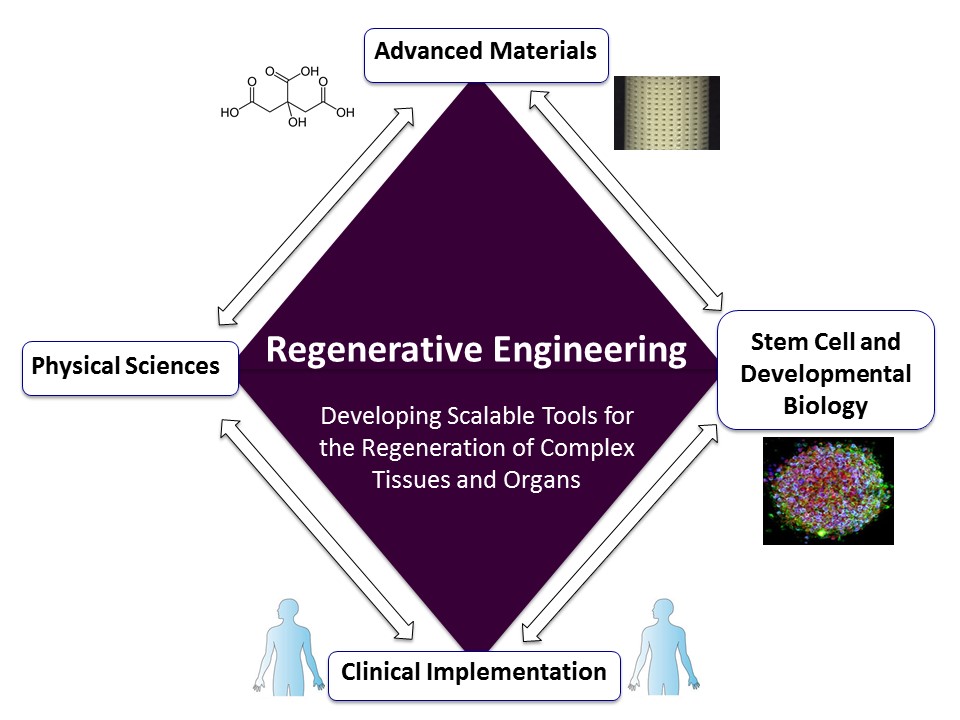
Regenerative engineering will require very close collaborations among scientists and engineers from several fields. Credit: Guillermo Ameer.
One of the greatest challenges that patients and surgeons often face is the body’s inability to jump-start the regeneration of injured, missing, or diseased tissue. The current gold standard is to obtain donor tissue from other parts of the body and transplant that tissue to the surgical site. Problems with this approach include: 1) a new defect is created at the donor site, which is often the source of pain, discomfort, and disfigurement, 2) lack of available quality donor tissue, and 3) depending on the tissue, multiple surgeries may be required, which significantly increases healthcare costs. Another approach is to use donor tissue or organs from a different individual. However, the number of available organ donors is significantly lower than the number of patients waiting for a transplant and tissue obtained from cadavers does not perform as well as living tissue. Furthermore, the recipient must be on lifetime immunosuppression to prevent rejection of the donated tissue or organ, which puts him or her at risk of complications such as chronic infections and cancers.
The field of regenerative medicine emerged as an attempt to address some of these problems by investigating the use of stem and progenitor cells from a variety of sources as well as specialized proteins that promote cell proliferation or migration (e.g. growth factors) to help tissues and organs regenerate. However, it is now clear that cell- or growth factor-based therapies alone will likely not be the panacea that many were hoping for and new strategies are required.
The promise of cost-effective tissue and organ regeneration that is available to all patients will require more complex solutions that can only be obtained through collaborations across several disciplines and the development of new fields that are focused on scaling up the most promising solutions for implementation. To this end, regenerative engineering is a new field defined as the convergence of advanced materials science, developmental biology (including stem cells technology), physical sciences, and clinical translation, to develop scalable and reliable tools that enable the regeneration or reconstruction of complex tissues and organs.
Therefore, regenerative engineering can be viewed as the field that aims to readily enable regenerative medicine so that it can become a practical option for the patient. Due to the involvement of advances in materials science such as nanotechnology and smart biomaterials to help guide or diagnose tissue regeneration and function, regenerative engineers will face many difficulties, the largest being the regulatory path that must be navigated by institutions and companies to make regenerative products available to the end user. The regulatory path dictates the expected safety, efficacy, and cost that it will take to implement these next-generation medical products in the clinic. A complex solution that involves cells, biologics (proteins or DNA), and new materials will lead to a more complicated regulatory path. Nevertheless, in the foreseeable future, new materials will be an essential component to the goal of tissue regeneration.
Developing the right biomaterials
Biomaterials for regenerative engineering can come in many compositions and forms. They are generally used as a biodegradable three-dimensional scaffold to guide or inform the regenerative process. They must also be amenable to the handling characteristics that are required by the surgeon to implant or deliver the product. Biomaterials must also meet the quality systems requirements for manufacturing medical devices. Despite the plethora of biomaterials that are reported in literature, the vast majority of biodegradable medical products that are on the market are based on decades-old polymer technology that was originally introduced to fabricate biodegradable or resorbable sutures— namely lactide or glycolide-based polymers. This is due to the need for companies to be as conservative as possible to facilitate the regulatory approval of new products and reduce costs. Natural tissues that have been processed to remove constituent cells or other components have also come to market.
However, many of these currently used materials do not provide the properties or requirements that regenerative engineering demand. Examples of how the development of new biomaterials with advanced functionality can impact the field of regenerative engineering and regenerative medicine are described below.
As most tissues and organs in the body are elastic and it is now recognized that cells and tissues respond to mechanical cues, it makes sense that a key property for a biomaterial that will be used as a scaffold to regenerate tissue is tunable elasticity. In addition, biomaterials must be compatible with cells and blood and not induce chronic inflammation due to their presence.
Another important property is the capacity of the biomaterial to be resorbed by the body as the new tissue develops without having a negative impact on the new tissue. To address these challenges, our research group pioneered the use of citric acid to develop new antioxidant biomaterials referred to as citrate-based biomaterials (CBB) that meet the aforementioned requirements. Citric acid is produced within our body and has been extensively used for many decades by people in the food, cosmetic, plastics, and pharmaceutical industries. It is readily available and considered an inexpensive commodity chemical. The chemical structure of citric acid enables this molecule to form several bonds with other molecules, such as alcohols and amino acids, to create a physical network. The choice of alcohol and the number of bonds that are formed during the CBB synthesis process dictate whether the resulting material is liquid or solid, tough or soft, elastomeric or stiff, degradable or not. CBBs can be fabricated at low temperatures without the need for catalysts, which can render a material or device toxic if the catalyst is not completely removed. The mechanical, optical, and degradation properties of the resulting CBB can be easily tuned, allowing for tremendous versatility as a tool to fabricate scaffolds for regenerative engineering applications.
Combinations of these properties have enabled the use of CBBs in a variety of applications including healing of diabetic skin wounds and the regeneration or replacement of heart, blood vessel, bladder, pancreas, bone, cartilage, and ligament tissue. For example, our research group developed a CBB that was formulated to be a liquid at room temperature for easy application and can instantaneously change to a gel due to body temperature. The material was shown to accelerate the healing of diabetic wounds by slowly releasing a stem cell homing factor. This same CBB, which is capable of conforming to the shape and depth of a wound, regenerated skull bone by delivering to the defect site stem cells that were designed to become bone cells. A team at the Ann and Robert H. Lurie Children’s Hospital of Chicago lead by Dr. Arun Sharma, demonstrated that stem cells isolated from bone marrow and grown on a degradable antioxidant elastic CBB supported the regeneration of bladder tissue. The new tissue had nerve and blood vessel compositions that were similar to those of the healthy intact organ. In another study, our research group demonstrated that antioxidant CBBs can also help regenerate the inner blood-contacting cell layer found in blood vessels. This inner cell layer is important to prevent unwanted clotting and scar-like tissue from forming in the blood vessel.
In another study performed at the University of Toronto by Dr. Radisic and colleagues, CBBs that were engineered to have shape memory properties—that is change their shape when exposed to temperature changes—were shown to support the regeneration of heart tissue and function by delivering cardiomyocytes (heart cells) in a minimally invasive manner. Finally, our team has shown that CBBs can be formulated to be “inks” that can be used for 3D printing complex scaffold at high resolution (~10 microns). This capability was used to fabricate degradable vascular scaffolds that can one day be used as stents to keep blood vessels open and help the blood vessel’s wall better regenerate normal tissue to minimize future re-occlusions.
The new exciting field of regenerative engineering will require the development of innovative materials that can be scaled up and meet several criteria for functionality and regulatory approval. CBBs are a powerful example of how biomaterials science and engineering can play an important role toward that goal. The development of these materials will require very close collaborations among scientists and engineers from several fields in order to render them useful tools that will enable regenerative medicine applications.




