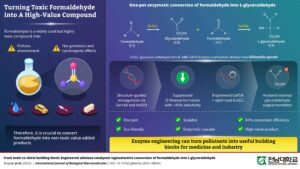
Structure-guided mutagenesis of fructose-6-phosphate aldolase enabled researchers to achieve 94% conversion of formaldehyde to L-glyceraldehyde in a one-pot aqueous process
Researchers at Chonnam National University in South Korea have engineered an enzyme cascade that converts formaldehyde into L-glyceraldehyde, a chiral compound used as a building block in pharmaceutical synthesis and in routes to specialty sugars. The one-pot process runs in water under mild conditions and reached roughly 94% conversion efficiency, pointing to a potential approach for detoxifying formaldehyde-containing waste streams while producing a higher-value chemical feedstock.
The study, published in the International Journal of Biological Macromolecules, lands amid growing interest in “C1 biocatalysis,” efforts to use enzymes and microbes to upgrade one-carbon molecules such as formaldehyde, methanol, formate, and CO₂ into multi-carbon products.
Why formaldehyde matters
Formaldehyde is widely used in manufacturing, including as a disinfectant and as a precursor for resins and other intermediates. It is also highly reactive and toxic. Exposure risks have made it a long-running occupational and environmental concern, and health agencies classify it as carcinogenic to humans.
Beyond direct industrial handling, formaldehyde can be generated in the atmosphere through chemical reactions and can off-gas indoors from building materials, furnishings, and consumer products made with formaldehyde-based resins, contributing to higher indoor concentrations in some settings.
Engineering the enzyme for selectivity
The Chonnam team used a structurally engineered fructose-6-phosphate aldolase (GaFSA) from Gilliamella apicola. The enzyme catalyzes carbon-carbon bond formation through an aldol reaction between glycolaldehyde and formaldehyde, yielding L-glyceraldehyde.
Early versions of the system produced substantial amounts of D-threose as a byproduct, cutting into selectivity for the target product. Using structure-guided mutagenesis, the researchers changed two residues (Ser166 and Val203) to suppress D-threose formation and improve selectivity to above 93% under aqueous, mild conditions.
To streamline the workflow, the team coupled the engineered aldolase with an optimized E. coli glyoxylate carboligase (EcGCL) to generate glycolaldehyde in situ from formaldehyde. That removed the need to add glycolaldehyde externally and enabled a single-vessel cascade.
The reaction operates at pH 7.5 and 40°C, proceeds entirely in water, and avoids organic solvents.
Part of a broader C1 biomanufacturing push
Formaldehyde sits in a precarious position for biomanufacturing. It is a useful intermediate for building carbon chains, but its toxicity can damage cells and deactivate biocatalysts. Reviews of C1 biomanufacturing have emphasized both the promise of turning low-value C1 streams into chemicals and the practical challenges in scaling these conversions.
Related research has shown that engineered whole-cell systems can convert formaldehyde into C2 products such as glycolic acid, a reminder that C1 upgrading is not limited to cell-free enzyme cascades.
What comes next
The results are an encouraging proof of concept, but several hurdles remain before any industrial rollout. Formaldehyde’s reactivity can shorten catalyst lifetimes, and long-run stability, regeneration strategies, and continuous-processing designs would all need development. Commercial viability would also depend on the cost and purity of available formaldehyde streams, the value of L-glyceraldehyde in downstream markets, and competition with established synthetic routes.
Still, the work adds to a growing toolkit for converting one-carbon feedstocks into more complex chemicals and suggests a future where at least some hazardous streams can be upgraded rather than simply neutralized.