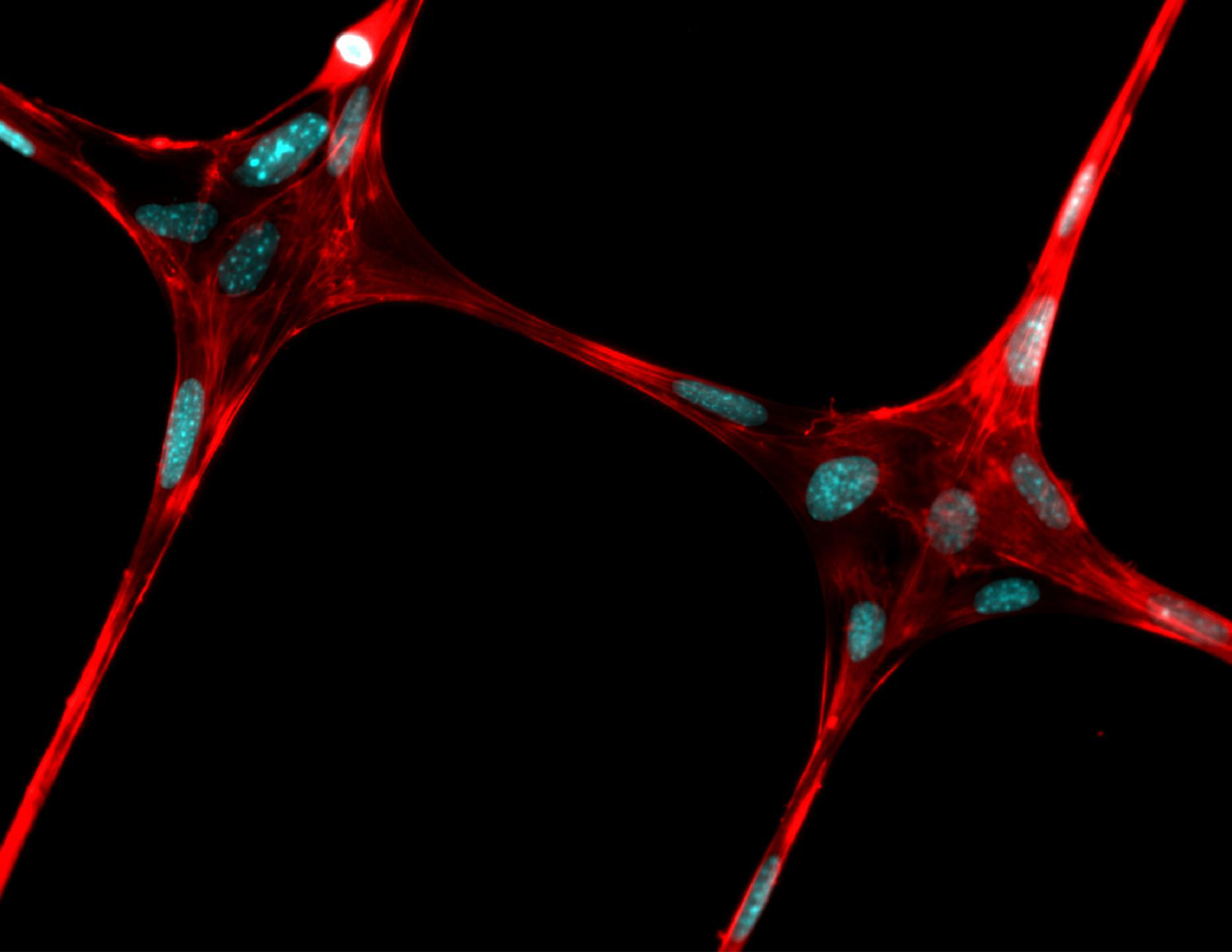
This is an illustration of electrospinning. Credit Justin Brown
Waiting on the donor list for a transplant could someday come to an end, as researchers have come one step closer to creating 3D printed, artificially grown body parts.
Engineers from Penn State University believe they have created the structural framework to grow living tissues using a combination of 3D printing and electrospinning—a method that uses electric charge to spin nanometer threads from either a polymer melt or solution.
“We are trying to make stem-cell-loaded hydrogels reinforced with fibers like the rebar in cement,” Justin Brown, an associate professor of biomedical engineering, said in a statement. “If we can lend some structure to the gel, we can grow living cells in defined patterns and eventually the fibers will dissolve and go away.”
Complex transplant tissues—including hearts, kidneys and tendons—currently come from either living or dead donors. However, this new method could produce a scaffold for tissue engineering that might also enable the production of combined muscles and tendons or tendons and cartilage.
“The overarching idea is that if we could multiplex electrospinning with a collagen gel and bioprinting, we could build large and complex tissue interfaces, such as bone to cartilage,” Pouria Fattahi, doctoral student in bioengineering, said in a statement. “Others have created these combination tissues using a microextrusion bioprinter.”
Current strategies create the different tissues separately, which are then combined using an adhesive or connector. However, in the body, tissues such as cartilage and bone, and tendons and muscles, grow seamlessly together.
The new apparatus uses the electrospinner to replace the extruder nozzle on the 3D printer so the printer can deposit a precise pattern of fibers in 3D to form a scaffold in a hydrogel, where cells can grow.
After the tissue is grown sufficiently, the scaffolding can be dissolved, leaving only a structured tissue appropriate for use.
When two different tissues are required, the printer can alter the pattern of threads so that the transition could be seamless with the appropriate cells, resulting in a naturally formed, two-part tissue replacement.
The overall goal is to create a novel, low-cost and efficient method to fabricate high-resolution and repeatable 3D polymer fiber patterns on nonconductive materials for tissue engineering with hobbyist-grade 3D printers.
The researchers are currently working on tissues that are less than one-inch cubes. They first produced exceptionally thin threads in the micron and nanometer range and then showed that they could grow cells on these fibers before finally depositing patterned fibers into a collagen gel loaded with cells.