Researchers from the Nanoscience Center at the University of Jyväskylä, Finland, have developed a computational model that could expedite the use of nanomaterials in biomedical applications. Their machine learning framework is capable of predicting how proteins interact with ligand-stabilized gold nanoclusters, materials widely used in bioimaging, biosensing and targeted drug delivery.
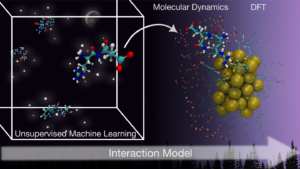
By integrating machine learning with atomistic simulations, the researchers developed a new interaction model that reveals the chemical rules guiding protein binding on gold nanoclusters. Image: Brenda Ferrari.
Gold nanoclusters are used in bioimaging for their natural fluorescence. For example, the material can be coated with molecules that are attracted to cancer cells, allowing doctors to determine where a tumor is. Gold nanoclusters designed to change color upon contact with proteins are also used to detect biomarkers. Unlike most nanoparticles, gold nanoclusters are small enough to pass through the kidneys, exiting the body in urine, making them safer than other nanoparticles.
A common approach to examine protein-nanoparticle interactions is molecular dynamics simulations, which can be challenging for computational modelling due to the complexity of interactions between each particle and between the particles and the environment.
“While the MD simulation of all these structures is feasible, it is an expensive venture, whereas for peptides with more than four amino acids, this is not a feasible approach. The application of machine learning techniques appears to be a promising approach to address this problem,” the researchers wrote in their paper, published in the journal Aggregate.
Existing studies that predict how proteins interact with nanoparticles often focus on isolated cases, leaving researchers without a unified predictive model to guide design. To address this, researchers at the Nanoscience Center developed a clustering-based machine learning framework that identifies the chemical principles involved in biomolecule adsorption on gold nanoclusters.
“The model determines which amino acids have higher or lower preference to bind to gold nanoclusters and identifies the specific chemical groups responsible for these interactions,” Brenda Ferrari, a postdoctoral researcher at the university, said in a press release.
The framework can scale beyond peptides, offering broader insights into protein-gold nanocluster interactions, potentially accelerating screening processes. With the model, the design of protein-gold nanocluster compounds could become more efficient, as it will become faster to screen proteins for specific functions or properties.
“Our goal was to build a model that doesn’t just explain one particular system, but that can be generalizable,” says Ferrari. We will continue working on the limitations, but we already have a model that can be extended to broadly explain protein–gold nanocluster interactions and support the development of smarter nanomaterials for biomedical use, she continues.


Tell Us What You Think!
You must be logged in to post a comment.