Traditional CRISPR-Cas9 methods have revolutionized genetics, but large functional sections of DNA, called payloads, still cannot be inserted into the genome without compromising cell viability or creating unwanted effects. Standard Homology-Directed Repair (HDR) dramatically loses efficacy for payloads exceeding 1 kilobase, compromising cell viability and increasing off-target edits.
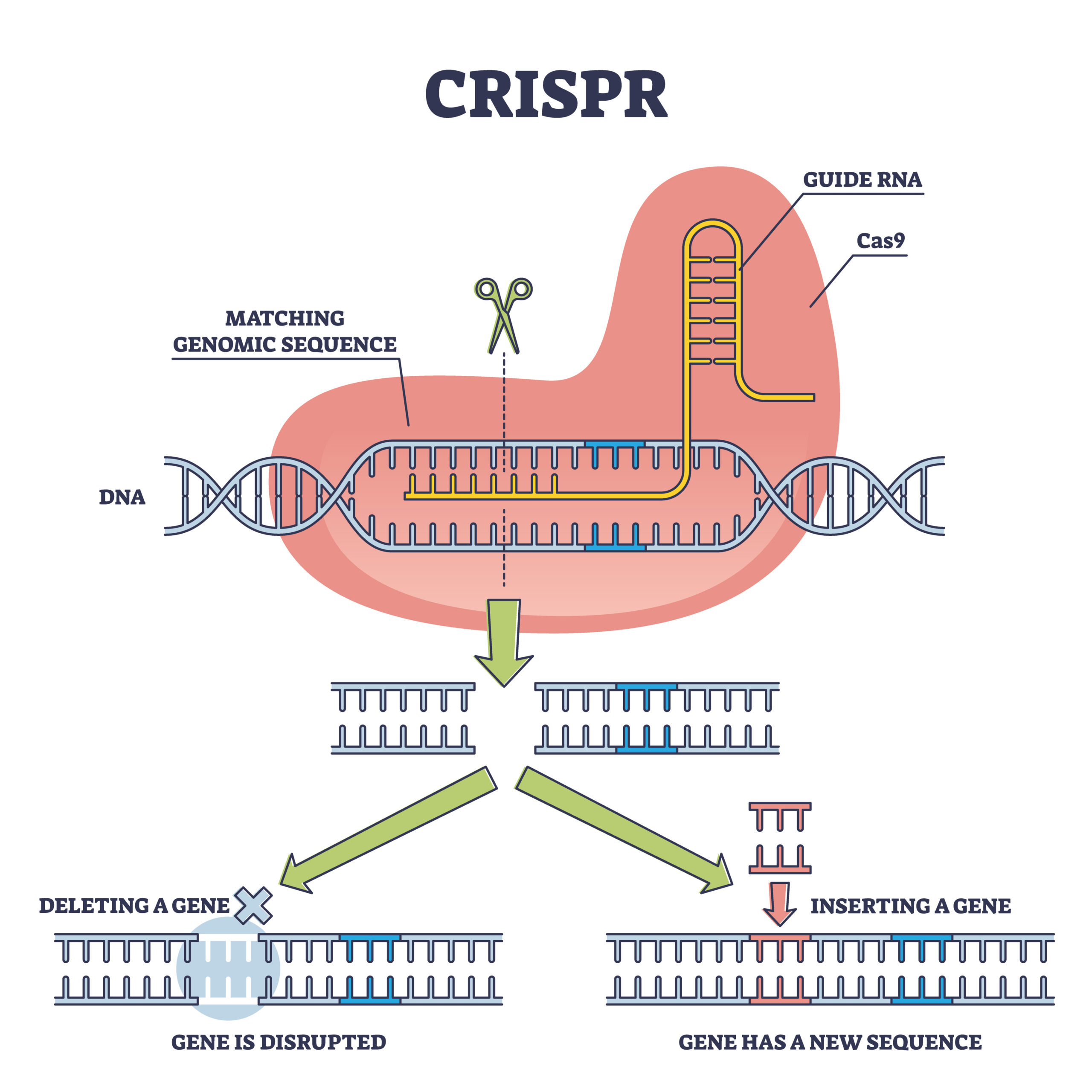
CRISPR-Cas9 methods use Cas9 to cut the DNA. Adobe
The 1 kb problem
CRISPR, which stands for “clustered regularly interspaced short palindromic repeats,” allows scientists to modify DNA. It is a natural defense mechanism found in bacteria and consists of DNA sequences derived from DNA fragments from a bacteriophage that infected the host, allowing the bacteria to “remember” past viruses and respond more effectively.
CRISPR-Cas9 is a common method for gene editing that uses the Cas9 enzyme to cut DNA. This method can edit multiple genes at once, making it efficient and cost-effective. However, it cannot efficiently integrate DNA payloads larger than 1 kb.
A full human gene is, on average, 62 kb including introns, meaning that Cas9 methods struggle to replace a full gene in order to correct a disease such as cystic fibrosis that is caused by mutations in a single gene.
Now, researchers have developed a Recombinases (Redα/β)-enhanced DNA integration technology called RED-CRISPR that delivers a two- to fivefold increase in “knock-in” efficiency for large DNA cargos compared to Cas9-HDR methods.
How recombinases achieve 5x higher efficiency
Recombinases (Redα/β) are recombination enzymes that enable functions such as excision and insertion, inversion, translocation and expression of genes. In RED-CRISPR, the Cas9 creates the precise DSB, the landing site, and the recombinases thread the donor DNA into the break site.
When engineering CAR-T cells, a cell therapy for cancer, RED-CRISPR demonstrated an insertion efficiency of up to 45%. Current commercial CAR-T relies on viral vectors which insert the gene randomly, creating a major safety risk. RED-CRISPR allows for site-specific integration, making the CAR-T cell treatment much safer.
When inserting a complex 8 kb payload into mouse embryonic stem cells, RED-CRISPR achieved a 43% knock-in rate for generation of genetically modified mice. Comparatively, Cas9 methods have a knock-in rate of approximately 15% for payloads larger than 1 kb.
The method also showed a reduction in chromosomal translocation — which can lead to cancer — and off-target editing, making the edits cleaner and more stable.