Delivering medications safely and accurately is of great interest to researchers and, of course, to people who need them. So is restoring function to damaged body parts. Jin K. Montclare, an associate professor of chemical and biomolecular engineering at the NYU Tandon School of Engineering, has taken a big step towards meeting both of these goals.
Her lab recently developed a protein polymer — specifically a protein-engineered triblock copolymer — that can self-assemble into hydrogels, absorbent networks of natural or synthetic polymer chains. As detailed in an article published in Biomacromolecules, these materials can then be used to get drugs to a targeted body part, such as a knee; and in regenerative medicine and tissue engineering, which uses cells, engineering, and biochemicals to improve or replace human cells, tissues, or organs.
The most notable part of her work, she says, “is trying to form hydrogels. It’s not a trivial feat.” Scientists previously succeeded in making hydrogels from synthetic polymers; in contrast, those engineered in the Montclare Lab for Protein Engineering and Design are derived from nature and are biodegradable. Engineered protein hydrogels have many potential advantages: they’re excellent biomaterials because they are flexible in form and size, and in the case of Montclare’s hydrogels, they can bind to a small molecule or drug, thus protecting it from degradation.
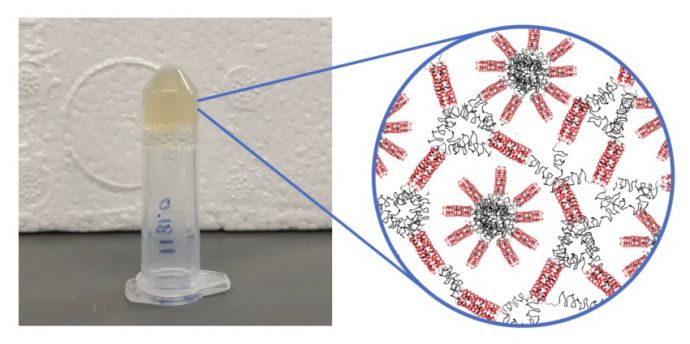
An inverted test tube holds the new soft protein hydrogel derived from nature. The drug-binding regions are in red, and the stimuli-responsive portion is shown in black; it can be used for a variety of biomedical applications.
After making the proteins, Montclare added curcumin, a naturally occurring chemical compound found in turmeric, which may reduce swelling and help ease pain and inflammation, as well as serve to combat cancers. The ingredients then self-assembled into a gel that encapsulated the curcumin.
“Ours are the first protein hydrogels with designed mechanical integrity to be able to specifically encapsulate drugs — getting them closer to use in biomedical applications,” Montclare says. Because they are soft gels, they are potentially injectable and can be infused with therapeutic agents or cells that might replace surgery and alleviate pain. “This could revolutionize treatment,” she adds. “That’s years down the line, but that’s where my mind is heading.” Hydrogels could be especially useful in repairing damaged cartilage, a painful effect of osteoarthritis, which affects about 30 million Americans, a number expected to more than double by the year 2040.
Another potential use for protein hydrogels is in gene therapy, where normal genes replace missing or defective ones as a way to correct genetic disorders. The hydrogels could possibly trap genes and nucleic acids and use these to treat genetic problems. That will take more research, Montclare notes, because the material they currently work with is neutral and would have to be positively charged to interact with the negatively charged nucleic acid for the gene therapy to work.
Her lab’s next steps include culturing specific types of cells to see if they can be used for scaffolds to grow particular tissue. The goal is to be able to grow gels to be compatible in firmness or mechanical integrity with the body part they are aimed at, such as cartilage.
Source: NYU Tandon School of Engineering




