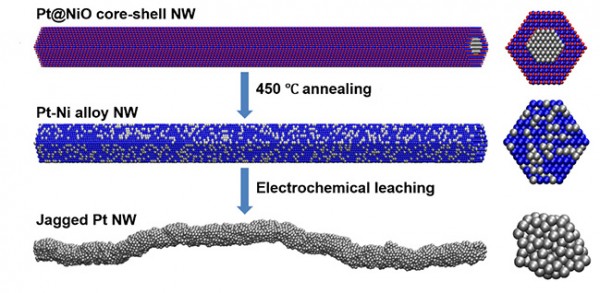
More active sites and more surface area on catalysts speed the chemical conversion of oxygen and hydrogen into water and electrons, generating electricity. Scientists devised a new synthesis route to produce a catalyst that doubles the conversion rate compared to the best previously reported catalyst. The process begins with a chemically synthesized thin wire with a platinum (Pt) core and a nickel oxide (NiO shell) (top). The team heats the wires to form a platinum-nickel nanowire (NW) (middle).The team electrochemically treats the wire to remove the nickel—resulting in a platinum wire with a jagged surface (bottom). Credit: Image courtesy of Dr. Xiangfeng Duan, University of California, Los Angeles
The Science
Fuel cells make electricity through chemical reactions. A key reaction is combining oxygen with hydrogen to make water while releasing energy in the form of electrons. The rate of this conversion is typically slow. It requires the presence of a catalyst such as platinum. In this research, a team developed a leaching process to produce ultrafine jagged platinum nanowires. The wires have with extraordinary surface activity and high surface areas. Combined, these features deliver a catalyst with a record-high conversion rate.
The Impact
The findings offer a new strategy for the design of highly efficient platinum-based catalysts. Such catalysts can dramatically reduce the amount of expensive platinum needed. These catalysts can lower the cost of fuel cells.
Summary
Platinum is an essential element for catalyzing the oxygen reduction reaction critical for fuel cell operations, a technology that generates electricity from chemical reactions of hydrogen and oxygen. The high cost of platinum is a primary factor that limits the adoption of electricity-generating fuel cells. A measure of the efficiency of the platinum catalyst is the mass activity—the catalytic activity divided by the weight of platinum. Higher mass activities must be achieved to reduce the required platinum usage and lower fuel cell costs. Improving the platinum mass activity requires optimizing both the specific activity and the electrochemically active surface area of the catalyst. Researchers at the University of California, Los Angeles, found that that they could convert nanowires with a platinum core and a nickel oxide shell, made by solution synthesis techniques, into platinum-nickel alloy nanowires through a thermal annealing process. The team could then transform the wires into jagged platinum nanowires via electrochemical dealloying or leaching. The jagged nanowires exhibit a mass activity of 13.6 amperes per milligram of platinum, which is nearly double the best previously reported values. Reactive molecular dynamics simulations (a type of computer modeling of the material) suggest that the highly stressed, under-coordinated surface structures enhance the desired reaction more than the relaxed surfaces of other platinum catalyst structures.




