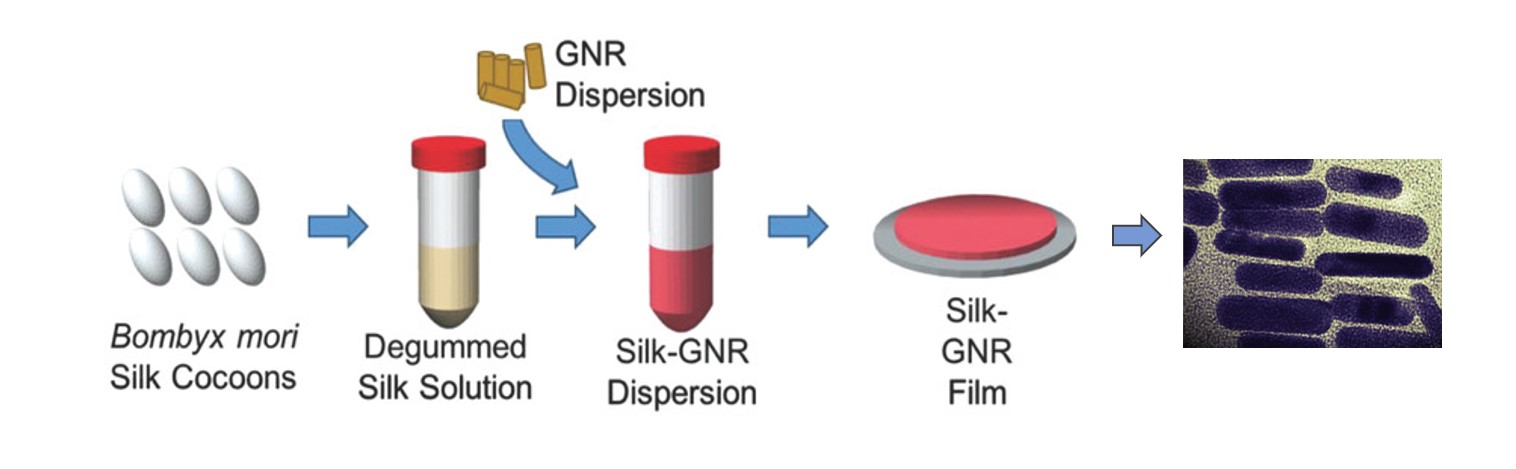
Sealant processing requires isolation of silk from cocoons, creation of silk solution, and addition of gold nanorods (GNR). The silk-GNR mix is formed into a silk-GNR film. The gold nanorods dispersed in the silk film are shown on the right. Image: Urie et al. Adv. Funct. Mater., 2018
NIBIB funded researchers have developed laser-activated nanomaterials that integrate with wounded tissues to form seals that are superior to sutures for containing body fluids and preventing bacterial infection.
Tissue repair following injury or during surgery is conventionally performed with sutures and staples, which can cause tissue damage and complications, including infection. Glues and adhesives have been developed to address some of these issues but can introduce new problems that include toxicity, poor adhesion, and inhibition of the body’s natural healing processes, such as cell migration into the wound space.
Now, researchers funded by NIBIB at Arizona State University are developing a novel sealant technology that sounds a bit like science fiction — laser-activated nanosealants (LANS).
“LANS improve on current methods, because they are significantly more biocompatible than sutures or staples,” explains David Rampulla, Ph.D., director of the NIBIB program in Biomaterials.
“Increased biocompatibility means they are less likely to be seen as a foreign, irritating substance, which reduces the chance of a damaging reaction from the immune system.”
However, biocompatibility does not imply simplicity. The Arizona group has developed this technology by carefully choosing and testing the materials contained in the sealant as well as the specific type of laser light needed to activate the sealant without causing heat-induced collateral tissue damage.
The sealant is made of biocompatible silk that is embedded with tiny gold particles called nanorods. The laser heats the gold nanorods to activate the silk sealant.
Once activated, the silk nanosealant has special properties that cause it to gently move into or “interdigitate” with the tissue proteins to form a sturdy seal. Gold was used because it quickly cools after laser heating, minimizing any peripheral tissue damage from prolonged heat exposure.
Two types of disc-shaped LANS were developed. One is water-resistant for use in liquid environments, such as surgery to remove a section of cancerous intestine. The sealant must perform in a liquid environment to reattach the ends of the intestine.
A leak-proof seal is critical to ensure that bacteria in the intestine does not leak into the bloodstream where it can result in the serious blood infection known as sepsis.
The water-resistant LANS were tested for repair of samples of pig intestine. Compared with sutured and glued intestine, the LANS showed superior strength in tests of burst pressure, measured by pumping fluid into the intestine.
Specifically, the LANS’ ability to contain liquid under pressure was similar to uninjured intestine and seven times stronger than sutures. LANS also prevented bacterial leakage from the repaired intestine.
The other type of LANS mix with water to form a paste that can be applied to superficial wounds on the skin. This type was tested on the repair of a mouse skin wound and compared to both sutured skin and skin repaired with an adhesive glue. The LANS were made into a paste, applied to the skin cut and activated with the laser around the margins of the sealant.
Two days after application, the LANS resulted in significantly increased skin strength compared to the glue or sutures. In addition, the skin had fewer neutrophils and cellular debris, which indicate that there was less of an immune reaction to the LANS.
“Our results demonstrated that our combination of tissue-integrating nanomaterials, along with the reduced intensity of heat required in this system is a promising technology for eventual use across all fields of medicine and surgery,” says Kaushal Rege, Ph.D., Professor of Chemical Engineering at Arizona State and the senior author of the study.
“In addition to fine-tuning the photochemical bonding parameters of the system, we are now testing formulations that will allow for drug loading and release with different medications and with varying timed-release profiles that optimize treatment and healing.”
The study was published in Advanced Functional Materials. The work was supported by grant EB020690 from the National Institute of Biomedical Imaging and Bioengineering, and the Department of Defense, Air Force Office of Scientific Research.
Source: National Institutes of Health


