Next-generation sequencing (NGS) tools have been a game-changer in the halls of drug discovery, where rapid and affordable sequencing has enabled faster characterization of samples, the ability to interrogate individual cells, and more frequent monitoring of cell lines for genetic drift, among other capabilities. While these platforms have contributed to significant advances in drug discovery…
New Applied Biosystems TaqMan QSY2 probes enable multiplexing of research
Biopharma assay developers and clinical research labs are under pressure to save time and reduce costs while assuring accuracy and delivering reproducible results. This can be accomplished by maximizing the number of targets per sample using multiplexing. The new Applied Biosystems TaqMan QSY2 probes* from Thermo Fisher Scientific demonstrate better assay performance retention when transitioning…
Forecast 2024: Pharma industry grapples with regulatory hurdles and AI opportunities
Heading into 2024, the pharma industry finds itself in uncharted waters. On the one hand, the sector’s commitment to R&D investment and innovation output is likely to remain strong, with tools based on AI, automation, and real-world data promising more streamlined drug candidate optimization. Additionally, the pharma sector is typically resilient in economic downturns. Financial…
Speedata’s chip accelerates complex pharmaceutical workload from 90 hours to 19 minutes in simulation
Speedata, whose first-of-its-kind Analytics Processing Unit (APU) is designed to accelerate big data analytic workloads across industries, has announced the results of a simulation of its APU on a compound similarity analysis workload in the pharmaceutical industry. Using the APU, the analysis was completed in 19 minutes, compared to 90 hours when using a CPU…
Cloud-based digital pathology platform helps CRO deliver more informed preclinical studies
Proscia, a provider of digital and computational pathology solutions, has announced that Virscio, a provider of translational research and development services, has deployed Concentriq for Research. The contract research organization (CRO) is leveraging Proscia’s flagship software platform to provide faster, more informed drug safety insights, helping its sponsors to improve research and development (R&D) efficiency.…
Protecting drug safety with forced degradation data management
By Jesse Harris, Digital Marketing Coordinator, ACDLabs In September 2019 the FDA learned that the common heartburn products Zantac and Axid contained elevated levels of the carcinogen N-nitrosodimethylamine (NDMA). Ranitidine and nizatidine, the active ingredients in these medications, appeared to be the source of this impurity. By April 1, 2020, the FDA asked that all…
Thermo Fisher Scientific expands France hub to get drugs from development to commercial manufacturing quicker
Thermo Fisher Scientific is adding early development work for oral solid dose therapies to its Bourgoin, France site, enabling that location to address customers’ workflow from early drug development through commercial manufacturing and underscoring the company’s commitment to helping clients get medicines to patients sooner. “In the last decade, Thermo Fisher has led and advanced…
Thermo Fisher Scientific opens facility at UCSF to accelerate development of breakthrough therapies
Thermo Fisher Scientific and the University of California, San Francisco (UCSF), will accelerate advanced cell therapies for difficult-to-treat conditions, including cancer, rare diseases, and other illnesses, from a newly opened cGMP manufacturing facility adjacent to UCSF Medical Center’s Mission Bay campus. The partnership between Thermo Fisher and UCSF, first announced in 2021, has the potential to demonstrate…
Waters’ next-generation HPLC system is aimed at reducing lab errors by up to 40%
Waters Corporation introduces Alliance iS, the next-generation intelligent HPLC System, designed to reduce compliance risk by adding new levels of proactive error detection, troubleshooting, and ease of use. When combined with Waters’ compliance-ready Empower Chromatography Software and eConnect HPLC Columns, the Alliance iS High Performance Liquid Chromatography (HPLC) System streamlines the task of making accurate…
Proscia announces expansion in preclinical R&D to drive faster drug safety decisions
Proscia, a provider of digital and computational pathology solutions, has strengthened its position in the $12 billion[1] preclinical R&D market. At the center of its expansion, the company is enhancing its flagship Concentriq for Research, trusted by 14 of the top 20 pharmaceutical companies as a singular software platform for pathology workflows and data, to…
Imaging agent illuminates lung cancer tumors
From Purdue University Surgery, especially surgery to remove cancerous tumors, relies on a range of tools and techniques as well as on the skill of the surgeon. Now, new imaging agent Cytalux will make surgery to remove lung cancer tumors a little more exact. The inside of the human body famously looks nothing like an…
ViroStat releases latest infectious disease catalog
ViroStat has just released its new catalog of infectious disease antibodies and antigens. This newest release includes 790 antibodies for use in antigen detection assays such as lateral flow rapid tests, ELISA, IFA, and CLIA. Many recommended pairs are available. Infectious targets include viruses, bacteria, toxins, parasites, and fungi. Go to the ViroStat website www.virostat-inc.com…
The promise and pressures of biologics R&D
By Christian Olsen, Business Segment Lead, Dotmatics Biologic drugs have increasingly delivered treatments for cancer and rare diseases and continue to prove effective in other wide-ranging areas, from neurological and metabolic disorders to respiratory and cardiovascular diseases. But along with the incredible potential biologics offer, there is also great pressure to discover and deliver novel…
The role of artificial intelligence in drug discovery
By Haydn Boehm, Director of Product Marketing, Dotmatics Despite the buzz around artificial intelligence (AI), most industry insiders know that the use of machine learning (ML) in drug discovery is nothing new. For more than a decade, researchers have used computational techniques for many purposes, such as finding hits, modeling drug-protein interactions, and predicting reaction…
R&D 100 winner of the day: Nortis ParVivo Platform
Nortis is an innovator in the rapidly emerging Organ-on-Chip (OOC) field, which enables the engineering of living human tissues in microfluidic devices (chips). These chips are highly valuable for both pharma and academia, both of which are looking for novel technologies that will enable them to move away from animal models to improve drug development…
Software platform helps researchers accelerate life-improving therapies
Dotmatics, a maker of R&D scientific software that connects science, data and decision-making, announces the release of its Small Molecule Drug Discovery Solution, an integrated scientific R&D platform with pre-configured workflows and expanded data management capabilities. The solution helps companies innovate more easily and expedites time to deployment by leveraging best practices derived from the…
Pharma’s top 20 R&D spenders in 2021
If 2020 underscored the importance of the pharma industry’s social importance, 2021 demonstrated the industry’s ability to translate R&D into profitable commercialized products. But while strong demand for COVID-19 therapies in 2021 provided unprecedented revenue levels for a handful of Big Pharma companies, it didn’t result in substantial changes in R&D spending. (Look out for…
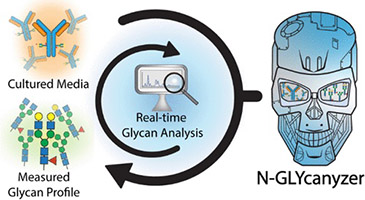
Rutgers pair creates monitoring toolkit to speed production of biologic drugs
Two Rutgers engineers specializing in the process of making drugs derived from living organisms have created an analytical tool they expect will accelerate the discovery and production of biologic drugs that are often at the cutting edge of biomedical research. In an article that is the cover story in the American Chemical Society journal, Analytical…
MilliporeSigma’s ZooMAb antibodies earns first-ever ACT Label from My Green Lab
MilliporeSigma, the U.S. and Canada Life Science business of Merck KGaA, Darmstadt, Germany, a science and technology company, has announced that its ZooMAb recombinant antibodies platform earned Accountability, Consistency and Transparency (ACT) label from My Green Lab, a non-profit organization dedicated to creating a culture of sustainability in science. The first-ever antibody to achieve ACT…
Rebuilding from the ground up: powering drug deformulation via analytical technologies
By Rob Taylor, Pharmaceutical Segment Manager, Malvern Panalytical Sometimes you must take things apart to rebuild them. For a child with a LEGO masterpiece, this can be heartbreaking. But in the world of pharmaceuticals, reverse-engineering drugs is crucial for the development of less expensive, more accessible generic products. Chemical and structural reverse engineering, also known…
PerkinElmer to showcase accelerated cancer research at AACR Annual Meeting
PerkinElmer. will be at The American Association for Cancer Research (AACR) Annual Meeting from April 8-13 in New Orleans (booths #1009, #1851 and #909), alongside its partner companies showcasing how genomic and cellular solutions for cancer research can be used to study its mechanisms, improve biomarker discovery, and help drive targeted, therapeutic discovery leading to…
Sepha’s new EZ Blister+ enables 21 CFR Part 11 compliant micro-batches
Sepha, the Northern Ireland-based manufacturer of pharmaceutical packaging and equipment, has developed a new compact small-batch blister packaging machine with full traceability and electronic data storage capabilities. The EZ Blister+ meets increased demand for 21 CFR Part 11 compliant micro-batches throughout the blister production lifecycle, from R&D right through to commercial production. It will launch…
R&D 100 winner of the day: Certara’s COVID-19 Vaccine Model
Certara’s COVID-19 Vaccine Model allows researchers to study how a vaccine is handled by the human body in computer-generated, virtual populations. It uses biosimulation (computer-aided modeling and simulation integrated with pharmaceutical and biological science) to generate virtual populations with different ages, gender, weight, genetics, diet, illnesses and medications. It can then efficiently run virtual clinical…
GenNext Technologies receives $2.5M grant to commercialize in-cell protein footprinting instrumentation
GenNext Technologies, a growth-stage company providing biopharmaceutical drug discovery systems and services, announced the receipt of a two year, Phase II SBIR grant for a project titled “In-Cell Automated Flash Oxidation (IC-AutoFox) Protein Footprinting System.” The grant was awarded by the National Institute of General Medical Systems (NIGMS) of the U.S. Department of Health and…
Genomenon raises $20M Series B financing, launching a new era in AI-powered genomic data
Genomenon Inc., an AI-driven genomics company, announces the completion of a $20 million Series B financing round. The funds will expand the company’s commercial operations and the development of its genomic data hub, which serves genetic testing labs, hospitals, pharmaceutical, and biopharma companies. Genomenon leverages AI (Artificial Intelligence) to organize the world’s genomic knowledge and…