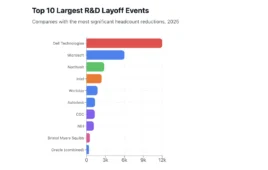
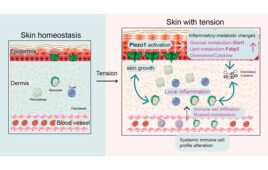
AstraZeneca announced that MedImmune, its global biologics research and development arm, has entered into a clinical trial collaboration with Advaxis Inc., a US-based biotechnology company developing cancer immunotherapies. The Phase 1/2 immunotherapy study will evaluate the safety and efficacy of MedImmune’s investigational anti-PD-L1 immune checkpoint inhibitor, MEDI4736, in combination with Advaxis’ lead cancer immunotherapy vaccine, ADXS-HPV, as a treatment for patients with advanced, recurrent or refractory human papillomavirus (HPV)-associated cervical cancer and HPV-associated head and neck cancer.
AstraZeneca announced that MedImmune, its global biologics research and development arm, has entered into a clinical trial collaboration with Advaxis Inc., a US-based biotechnology company developing cancer immunotherapies. The Phase 1/2 immunotherapy study will evaluate the safety and efficacy of MedImmune’s investigational anti-PD-L1 immune checkpoint inhibitor, MEDI4736, in combination with Advaxis’ lead cancer immunotherapy vaccine, ADXS-HPV, as a treatment for patients with advanced, recurrent or refractory human papillomavirus (HPV)-associated cervical cancer and HPV-associated head and neck cancer.Both MEDI4736 and ADXS-HPV are cancer immunotherapies, a new class of treatments that use the body’s own immune system to help fight cancer. MEDI4736 is designed to counter the tumor’s immune-evading tactics by blocking a signal that helps tumors avoid detection, while ADXS-HPV enhances the ability of immune cells to combat the tumor. Preclinical evidence suggests that the combination of ADXS-HPV with a checkpoint inhibitor, such as MEDI4736, can enhance overall anti-tumor response.
“Our collaboration with Advaxis is further evidence of MedImmune’s commitment to explore novel combination approaches as we progress our immuno-oncology portfolio,” said Dr. Bahija Jallal, executive vice president, MedImmune. “We believe there could be an important clinical benefit from the combination of MEDI4736 with Advaxis’s antigen-specific cancer vaccine.”
Under the terms of the agreement, MedImmune and Advaxis will evaluate the combination as a treatment for HPV-associated cervical cancer and squamous cell carcinoma of the head and neck. The Phase 1 part of the trial is expected to establish a recommended dose regimen of MEDI4736 with ADXS-HPV, and the Phase 2 portion will assess the safety and efficacy of the combination. The study will be funded and conducted by Advaxis. Results from the study will be used to determine whether further clinical development of this combination is warranted.
Under the terms of the deal, MedImmune has a non-exclusive relationship with respect to HPV-driven tumor types. MedImmune has first right of negotiation for future development of combinations involving MEDI4736 and ADXS-HPV.
“We are excited to be partnering with MedImmune and evaluating MEDI4736 in combination with our immunotherapy,” said Daniel O’Connor, chief executive officer, Advaxis. “This is the first time a PD-L1 checkpoint inhibitor will be used with a new class of immunotherapies. As multiple companies vie for a competitive advantage in the future PD-L1 market, the ability of our immunotherapy platform to attack multiple tumor targets makes it an attractive combination therapy.”
AstraZeneca and MedImmune have recently initiated other immuno-oncology combination trials, including a collaboration with biopharmaceutical company Incyte to study MEDI4736 with Incyte’s oral indoleamine dioxygenase-1 (IDO1) inhibitor, INCB24360.
Date: July 22, 2014
Source: AstraZeneca