Despite the fact that many types of drugs work by blocking a G-protein-coupled receptor (GPCR), there are currently no approved cancer drugs that work this way. The existing scientific literature includes published papers, which observe that dopamine receptors have an effect on cancer but this connection raises a few key questions.
1. Does it make sense that neurotransmitter pathways have a role in oncology?
Dopamine Receptor D2 (DRD2) is overexpressed by several types of human cancer and its inhibition is associated with anti-cancer activity. This is documented by studies describing: 1) elevated DRD2 expression in malignant cells compared with normal cells; 2) preclinical studies documenting the antitumor effects of antagonizing DRD2 signaling; and 3) observed associations of cancer incidence with other diseases that affect dopamine receptor signaling.
First, there is a large body of literature indicating elevated DRD2 expression in malignant cells compared with normal cells in several tumor types. Moreover, this overexpression tends to increase with the stage of the disease. This is consistent with finding that DRD2 impacts several signaling cascades that are intimately involved in cancer cell survival, angiogenesis, migration, and metastasis. See Table 1 for studies indicating elevated DRD2 expression in malignant cells compared with normal cells.
Second, there are a large number of preclinical studies documenting the antitumor effects of antagonizing DRD2 signaling, either through genetic or pharmacological inhibition (Table 2). The most thoroughly investigated is thioridazine, an antipsychotic DRD2 antagonist that in preclinical models is pro-apoptotic, anti-angiogenic, and depletes cancer stem cells in several tumor types. Several signaling pathways have been implicated in the antitumor mechanism of DRD2 inhibitors, including downregulation of Ras signaling and upregulation of oxidative stress.
Third, there are meta-analyses that have examined cancer incidence in populations with dysregulated dopamine receptor signaling and have found that dopamine blockade leads to lower levels of cancer. For example, a meta-analysis of patients with Parkinson’s disease (Bajaj et al, 2010), where dopamine is downregulated (effectively antagonized), revealed a significantly reduced incidence of most cancers. Interestingly, while this association was true for most solid and hematological malignancies, the exceptions – including melanoma and thyroid cancers – are tumors with low expression of DRD2. A similar analysis of schizophrenic patients where dopamine is upregulated, found an increase in cancer incidence that is reversed in patients who are compliant with treatments that antagonize dopamine (Dalton et al, 2005).
2. How is dopamine receptor signaling involved in cancer?
DRD2 is a member of the dopamine receptor family that is grouped into two classes: the D1-like receptor class composed of DRD1 and DRD5 and the D2-like receptor class composed of DRD2 (with the highest affinity for dopamine), DRD3, and DRD4. All of the dopamine receptors are G protein-coupled receptors (GPCRs), whose signaling is primarily mediated by interaction with and activation of heterotrimeric GTP-binding proteins (G proteins). GPCRs control pro-survival signaling pathways (e.g. ERK and Akt) that are broadly important in human cancer.
The D2-like receptor family is coupled to G specific types of G proteins that mediate their signaling. One type of G protein coupled to D2-like receptor activation acts as an inhibitor
of adenylate cyclase, a key enzyme involved in the synthesis of cyclic AMP (cAMP) that affects many intracellular signaling pathways. By contrast, the D1-like family of receptors is coupled to a different type of G protein that stimulates adenylate cyclase and thus opposes D2-like receptor signaling.
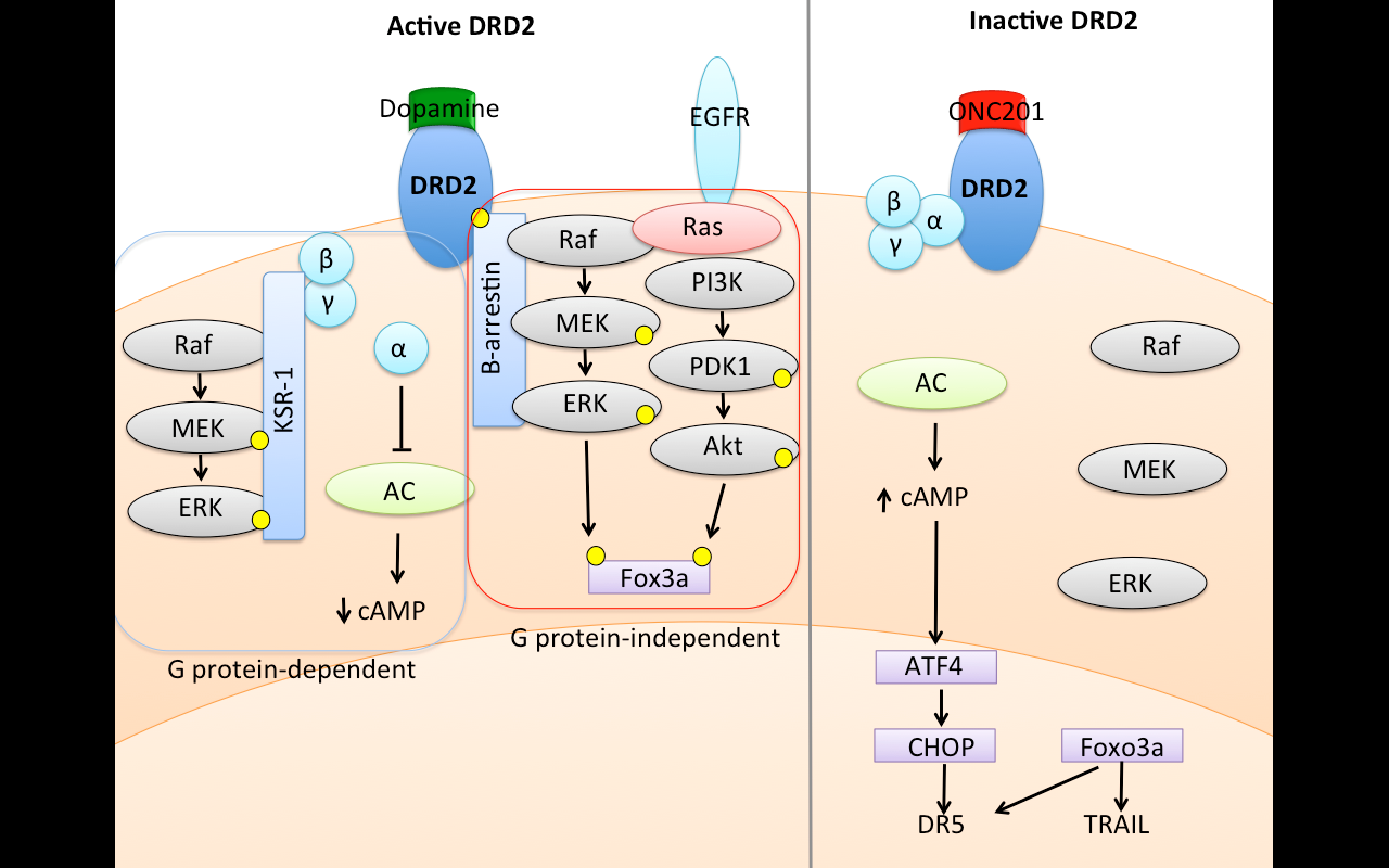
Figure 1. DRD2 signaling upon stimulation involves downregulation of cyclic AMP and upregulation of Ras signaling. Credit: Oncoceutics
Activation of D2-like receptors also stimulates ERK and Akt signaling that promotes cancer cell survival. The mechanism that couples D2-like receptors to this signaling is complex and differs depending on cell type, but often involves scaffold proteins such as KSR-1 or β-arrestin that are recruited to the activated GPCR and facilitate kinase signaling cascades.
Oncoceutics’ lead compound ONC201 has demonstrated significant anti-tumor activity in both pre-clinical and clinical studies dating back to 2011. Recently, it has been demonstrated that ONC201 is a selective antagonist of DRD2, a GPCR that is a dopamine receptor, and that this DRD2 antagonism is responsible for antitumor activity of ONC201.
This makes ONC201 the first clinical molecule for oncology that targets neurotransmitter pathways.
Table 1. Studies indicating elevated DRD2 expression in human cancers
|
Tumor Type(s) |
Conclusion |
Reference |
|
Small cell lung cancer |
Elevated plasma dopamine & dopamine receptor expression in SCLC |
Cherubini et al, 2016 |
|
Cervical cancer |
DRD2 is associated with cervical cancer progression; cervical cancer cells respond to thioridazine |
Mao et al, 2015 |
|
Neuroendocrine tumors |
DRD2 is expressed in 35% of GI neuroendocrine neoplasms |
Diakatou et al, 2015 |
|
Lung cancer |
DRD2 is expressed in 74% of lung carcinoids |
Kanakis et al, 2015 |
|
Meningioma |
DRD2 was expressed in 93% of meningiomas |
Trott et al, 2015 |
|
Pituitary adenoma |
DRD2 is the highest expressed dopamine receptor in pituitary adenomas |
Gabalec et al, 2015 |
|
Pheochromocytoma |
DRD2 mRNA & protein is expressed in pheochromocytomas |
Saveanu et al, 2013 |
|
Neuroendocrine tumors |
11/17 neuroendocrine tumors expressed DRD2 |
Pawlikowski et al, 2011 |
|
Pheochromocytoma/paraganglioma |
DRD2 is highly expressed in pheochromocytomas and paragangliomas |
Saveanu et al, 2011 |
|
Neuroendocrine tumors |
DRD2 was expressed in gastroenteropancreatic neuroendocrine tumors |
Diakatou et al, 2011 |
|
Cholangiocarcinoma |
Elevated Dopamine secretion and DRD2 expression |
Coufal M et al 2010 |
|
Neuroendocrine tumors |
DRD2 is expressed in the majority of low and intermediate grade neuroendocrine tumors |
Srirajaskanthan et al, 2009 |
|
Corticotroph adenomas |
DRD2 is expressed in corticotroph adenomas |
de Bruin et al, 2009 |
|
Colon cancer |
DRD2 genotypes can increase risk of colon cancer recurrence |
Murphy et al, 2009 |
|
Neuroendocrine tumors |
DRD2 is expressed in neuroendocrine tumors, more prevalent in aggrestive tumors |
Grossrubatscher et al, 2008 |
Table 2. Studies indicating anti-tumor efficacy of DRD2 antagonists in preclinical models
|
Tumor Type(s) |
Conclusion |
Reference(s) |
|
Solid tumors |
Trifluoperazine has anti-metastatic properties |
Pulkoski-Gross et al, 2015 |
|
Breast cancer |
DRD2 agonists or antagonists kill MCF7 cells |
Pornour et al, 2015 |
|
Glioblastoma |
Genetic knockdown or haloperidol has anticancer activity in GBM |
Li et al, 2014 |
|
Breast cancer |
Thioridazine and doxorubicin possess anticancer activity |
Ki et al, 2014 |
|
Neuroendocrine tumors |
Inhibition of DRD2 and STTR2 has anticancer effects in midgut carcinoid cells |
Zitzmann et al, 2013 |
|
Neuroblastoma |
Sertindole induces neuroblastoma cell death and autophagy |
Shin et al, 2012 |
|
Hepatocellular carcinoma |
DRD2 inhibitors possess anticancer activity |
Chen et al, 2011 |
|
Neuroblastoma |
Halopiredol possesses anticancer activity |
Gasso et al, 2012 |
|
Small cell lung cancer |
Cortexolone possesses anticancer activity |
Reina et al, 2011 |
|
Glioblastoma |
Eticlopride possess anticancer activity |
Visnyei et al, 2011 |
|
Prostate & NSCLC |
Inhibition of DRD2 and STTR2 has anticancer effects |
Arvigo et al, 2010 |
|
Cholangiocarcinoma |
DRD2 antagonism reduces dopamine-mediated tumor cell proliferation |
Coufal M et al 2010 |
|
Glioblastoma |
Thioridazine possesses anticancer activity |
Cheng et al, 2015 |
|
Cervical cancer |
Mao et al, 2015 |
|
|
Murine breast cancer |
Yin et al, 2015 |
|
|
Hepatocellular carcinoma |
Lu et al, 2015 |
|
|
Ovarian cancer |
Park et al, 2014; Rho et al, 2011; Byuen et al, 2012; |
|
|
Gastric cancer |
Mu et al, 2014 |
|
|
Nasopharyngeal carcinoma |
Lan et al, 2014 |
|
|
Breast cancer & leukemia |
Sachlos et al, 2012 |
|
|
Cervical & endometrial cancer |
Kang et al, 2012 |
|
|
Lymphoma |
Spengler et al, 2011 |
|
|
Glioma and neuroblastoma |
Gil-Ad et al, 2004 |
|
|
Lymphoma and leukemia |
Zhelev et al, 2004 |
|
|
Breast cancer |
Strobl et al, 1990 |




