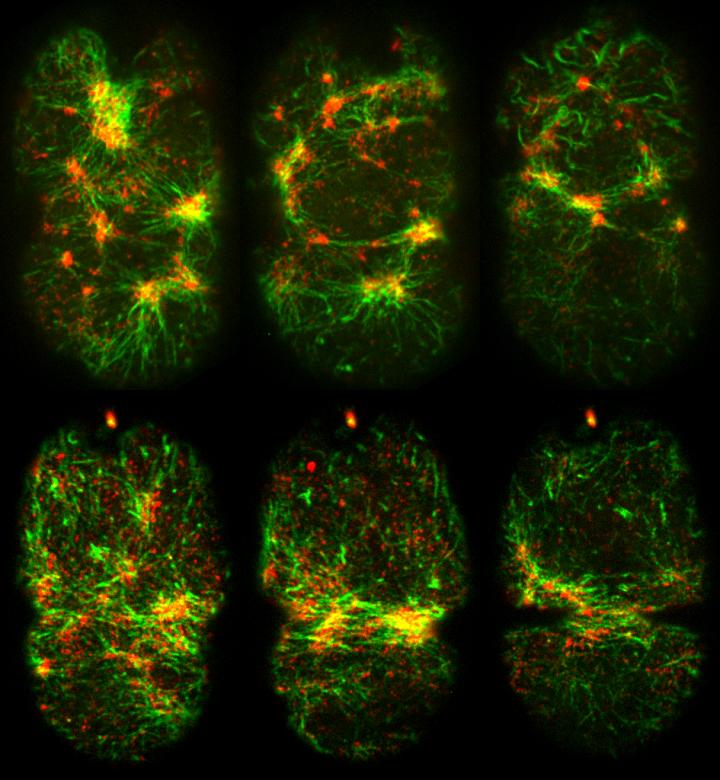
This figure shows plastin (green) and non-muscle myosin II (red) in the cortex of a newly fertilised C. eleganszygote during polarization (top panel) and cytokinesis (bottom panel) using spinning disk confocal microscopy. Co-localization between green and red signals signifies localized cortical contractility. Credit: Mechanobiology Institute, Singapore
Researchers from the Mechanobiology Institute, Singapore (MBI) at the National University of Singapore have described, for the first time, how plastin, an actin-bundling protein, acts as a molecular rivet, providing global connectivity to the cortex underlying the plasma membrane of embryonic cells to facilitate polarisation and cell division. The work was published in The Journal of Cell Biology on 11 April 2017.
Plastin promotes polarisation and cytokinesis in the C. elegans zygote
All multicellular organisms begin their life when a sperm cell fuses with an oocyte. The newly fertilised zygote must then undergo countless rounds of cell division as it forms an embryo. Some divisions are symmetric and generate two identical daughter cells in a process known as cytokinesis. Others are asymmetric, and result in unequal daughter cells that differentiate into distinct cell types with specialised functions. Before asymmetric division, these cells undergo a process known as polarisation, where the top or front of the cell contains a different set of protein-based structures and machines to the bottom or back of the cell.
Both cytokinesis and polarisation are driven by a contractile component of the cell called the cortex. This thin layer is located inside the cell, immediately adjacent to the plasma membrane and is primarily composed of actin filaments (F-actin), which are cable-like structures that are dynamically assembled and disassembled, and maintain cell shape. The cortex also contains the motor protein non-muscle myosin II, which confers contractility to the network, and other actin-binding proteins such those that bundle filaments together or facilitate the assembly of new filaments. Despite the structural arrangement of the cortex being well established, it was unclear how all of these proteins work together in a living organism to produce higher order actin-based structures, and transmit mechanical force during crucial developmental processes.
To investigate these questions, a team of interdisciplinary scientists from MBI, A*STAR’s Institute of Molecular and Cell Biology, and the European Molecular Biology Laboratory, examined the cortex in a developing organism, specifically, Caenorhabditis elegans (C. elegans), which is a 1mm long transparent nematode worm. What became apparent from the investigation, which was led by Assistant Professor Ronen Zaidel-Bar, was the important role of an actin-binding protein called plastin (a.k.a. fimbrin) in early C. elegans development.
Plastin binds to actin filaments to facilitate their bundling and strengthen the actin filament network so that it can withstand the forces generated during filament contraction, and also those applied to the cortex from external stimuli. By functioning as a molecular rivet, plastin enabled the cell cortex to function efficiently, thereby facilitating polarisation and cytokinesis. Using microscopy, genetic and computer modelling approaches, the researchers examined the cell cortex during the earliest stages of C. elegans development, with a particular focus on plastin. Although previous efforts had identified plastin within the cortex, none had revealed its full importance in the development of an embryo.
To begin the investigations, MBI PhD candidate Mr Ding Wei Yung examined zygotes from a mutant strain of C. elegans where the function of plastin had been lost. In these zygotes, both polarisation and cytokinesis were disrupted to the point that they either did not occur, or were significantly delayed. With the ability to observe how C. elegans formed with plastin mutated, the researchers turned their attention to examining contractility within the cortex, during C. elegans development.
To do this, the researchers examined the organisation and dynamics of non-muscle myosin II at the cortex during polarisation. In normal zygotes, the myosin motor proteins will accumulate into large clusters that generate large contractile forces. However, when plastin function was lost, the researchers noted that the myosin did not accumulate in clusters as it does in normal cells. This meant that the contractions generated were much weaker compared to normal cells. Ultimately, the loss of strong, coordinated cortical contractions in the plastin mutant worm embryo resulted in defective polarisation. This was evident from the disrupted separation of two proteins that would otherwise accumulate at either end of the cells as they underwent polarisation.
The authors then extended their investigation beyond polarisation and looked at the role of plastin in cytokinesis. In healthy zygotes, actin filaments, together with non-muscle myosin II, will accumulate in the middle of the dividing cells. From there they effectively pull the membrane inwards so that opposing sides of the cell meet and fuse, thereby creating two cells. This did not occur at the same rate in cells containing mutated plastin, and it was determined that plastin facilitates the accumulation of the proteins in the correct position.
To better understand why plastin-mediated cross-linking of actomyosin filaments had these effects on cell polarisation and cytokinesis, the team turned to mathematical modeling. Specifically, they tested whether increased connectivity between plastin and actomyosin alone is sufficient to drive long-range cortical contractility. These simulations revealed an optimal level of plastin crosslinking required to facilitate these processes, and indicated that too little or too much cross-linking will result in an F-actin network that is either too disconnected or too stiff. In both cases, the end result is weakened contractility. To confirm that this was true in living organisms, the team increased the levels of plastin in the healthy C. elegans zygotes, and found that indeed, having too much plastin substantially slowed down cytokinesis.
Embryogenesis requires robust force generation and transmission that drives critical cellular processes such as polarisation and cytokinesis. The findings presented in this work elucidate a role for plastin as a molecular rivet to facilitate robust polarisation and timely cytokinesis. These discoveries further demonstrate the importance of the high-order organisation of the actomyosin cytoskeleton and the role of actin binding proteins, such as plastin, in regulating its function.




